Linda D. Lee, DrPH, MS, MBA, Executive Vice President and Chief Science Officer, VidaShield
Corresponding Author:
Dr. Linda Lee, American Green Technology, 52129 State Route 933, South Bend, IN 46637
ABSTRACT
Background: Short-wave ultraviolet light (UV-C) is known to have the ability to render bacteria inert. We theorized that using UV-C in a continuous fashion at the room level would not only lower the amount of bacteria circulating in the air, but also lessen the amount of bacteria found on surfaces in the same space.
Methods: We set up field trials at three hospitals (Texas, Nevada, and Massachusetts) where we tested air and surface for bacteria, installed continuous UV-C products at the room level, and then tested air and surface again.
Results: In all cases, airborne bacteria was reduced between 79 and 91% over pre-installation values. Most surfaces also showed reductions in bacteria from 48 to 69%, although we report one incident of an increase of 288%.
Conclusion: The data indicate that using active air UV-C technology at the room level reduces the bioburden in the air and on surfaces, including in occupied spaces. Hospitals should consider implementing active UV-C technology to improve air quality.
KEYWORDS
Air disinfection, UV-C, airborne bacteria
INTRODUCTION
 An early publication on the effectiveness of ultraviolet light on bacteria is from 1877, when two British scientists noticed that Pasteur’s solution, when placed in lead-covered test tubes, grew innumerable bacteria, while the same solution in unshielded test tubes placed in sunlight, did not (1). Since then, many studies have demonstrated that UV rays are a powerful way to render bacteria inert, beginning with Coblentz in 1922 (2) and Sharp in 1939 (3).
An early publication on the effectiveness of ultraviolet light on bacteria is from 1877, when two British scientists noticed that Pasteur’s solution, when placed in lead-covered test tubes, grew innumerable bacteria, while the same solution in unshielded test tubes placed in sunlight, did not (1). Since then, many studies have demonstrated that UV rays are a powerful way to render bacteria inert, beginning with Coblentz in 1922 (2) and Sharp in 1939 (3).
It has been known for decades that many diseases, such as tuberculosis and influenza, are spread via airborne and/or droplet transmission. More recently, studies have shown that pathogens thought to be spread through direct contact can also become aerosolized. Roberts et al. demonstrated that Clostridium difficile (C. diff.) spores could be disseminated through the air (4) as did Best et al. (5). Li et al. reviewed 40 studies to show a strong association between building ventilation and the transmission of airborne disease (6). Eames et al. wrote similarly, but with a tighter focus on hospital acquired infection (HAI), including methicillin-resistant Staphylococcus aureus (MRSA) (7). Nazaroff’s discussion of indoor bioaerosol dynamics lays out how the airflow in a space moves particulate matter, including microbes (8).
 Knowing that disease could be spread through the air, and that short-wave ultraviolet (UV-C) can render pathogens inert, it is logical that the medical community would turn to UV-C to reduce the amount of bacteria circulating in the air. Bolton and Cotton discussed how UV disinfection works in general (9) and Boyce discussed specific technologies for using UV-C in hospitals (10).
Knowing that disease could be spread through the air, and that short-wave ultraviolet (UV-C) can render pathogens inert, it is logical that the medical community would turn to UV-C to reduce the amount of bacteria circulating in the air. Bolton and Cotton discussed how UV disinfection works in general (9) and Boyce discussed specific technologies for using UV-C in hospitals (10).
Rutala et al. studied how UV-C worked at the room level to eliminate bacteria (11).
Over the decades, several approaches to UV-C were developed. These methods included using UV-C as part of the water filtration system, using it in the HVAC system, and using it as a stand-alone, mobile product. Each method has some things to recommend it, in terms of effectiveness, ease of use, and cost, but also each one has drawbacks, including these same factors and, in the case of the mobile unit, the necessity for training as well as the requirement that the space to be treated be unoccupied. Reed provided an excellent historical perspective (12) and Memarzadeh et al. concluded that ultraviolet germicidal irradiation (UVGI) is a useful addition to the disinfection toolbox (13).
The potential for surfaces to hold onto microbial contaminants despite standard cleaning methods is clear. Hospodsky et al. noted that an important source of airborne materials is a result of human activity, such as entering a room, which resuspended particles from surfaces (14). Our study was designed to examine the effect of using UV-C at the room level on the amount of bacteria in the air, and whether cleaning the air would have a positive effect on surface bacteria.
METHODS
Environmental studies were conducted at an acute-care hospital in Massachusetts (Hospital A), an acute-care children’s hospital in Texas (Hospital B), and an acute-care hospital in Nevada (Hospital C). In each case, the study materials and methodology were the same. Baseline air and surface samples were taken, UV-C units were installed, and several weeks after that, air and surface sampling were repeated, and before-and-after results compared.
Baseline microbiologic sampling for the studies was accomplished by collecting air samples onto trypticase soy agar with blood (TSA) plates (Hardy Diagnostics, Santa Maria, CA). The sampler works by pulling air in through a perforated cover. The air impacts the agar plates, which are coated with blood. The cells that land on the plates start to reproduce and form colonies. These colonies are counted (raw CFU). This number is adjusted using a standard method for the probability that more than one viable particle was pulled though a single sampling hole and merged with other particles on the plate to produce a single colony. This adjustment is the correction hole factor.
Multiple samples were taken from each location. Representative areas sampled included next to the bed, at the window, near the linen cart, at the nightstand and near the window.
Air samples were collected with SAS 180 samplers (BioScience International, Rockville, MD). All air samples were run at 1000L (approximately five and a half minutes), and air was collected onto 90 mm sampling plates. As plates were collected, they were packed in coolers with gel packs, then packaged with gel packs and shipped overnight to an independent laboratory (Antimicrobial Test Laboratories (now Microchem Laboratories), Round Rock, TX).
Surface samples of 25 cm2 were collected directly onto the Rodac sampling plates, using a straight downward motion to insure the sampling plate contacted the surface with sufficient pressure to collect the sample. Plates were then refrigerated and prepared for overnight shipping to the lab. For surface bacterial sampling, TSA with Lecithin and Tween plates were used.
Multiple samples were taken from each location. Representative areas sampled included the bed rails, the over-bed table, keyboards and chair arms. All plates were refrigerated and prepared for overnight shipping to the same independent lab. At the lab, all plates were incubated at 30 ± 2° C for 5-7 days, after which they were evaluated. Total colony forming units (CFUs) were recorded for each specimen.
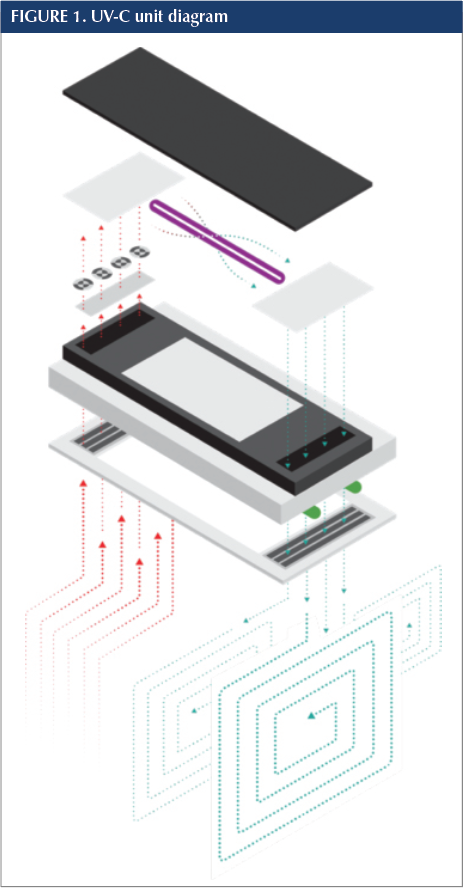
In each study location, after pre-installation sampling was complete, UV-C units (VidaShield™; American Green Technology, South Bend, IN) were installed. Each unit contained a fully shielded UV-C bulb housed atop a standard 2 x 4 ceiling light fixture. A 59 watt shielded UV lamp produced 15 watts of high output ultraviolet-C energy at a wavelength of 253.7 nanometers. Because the radiation chamber where the UV lamp is housed is enclosed and the air passes through the chamber, there is little to no distance from the lamp to the air that passes directly over the lamp. At its furthest point, the span is 6 inches. Each unit holds four small fans (similar to those in a desktop computer) that create differential pressure to continuously draw air into the system at 50 cubic feet per minute. On the way to the irradiation chamber, the air passes through a MERV 6 filter to remove dust and large particulates and then, once treated, the cleaned air is pushed back into the room. The intake and exhaust baffles are set at a 30-degree angle, which moves the air in a pattern that avoids repeatedly recirculating the same air. The fans draw air into the unit at a rate of 50 CFM. When operating continuously, the unit theoretically will treat a volume of air equivalent to an 8' x 10' x 10' (800 cubic feet) room four times per hour. The UV-C portion of the units run continuously, 24/7 whether the overhead room light is on or off. The units available for test were with no downlight, fluorescent/LED downlights and LED array downlights. Units were installed following each facility’s infection control risk assessment (ICRA). Once the units were operational all areas were reopened for normal use. The product used in this study is requires only minimal maintenance (an annual bulb and filter change), easily performed by existing facilities services staff.
For a variety of hospital operational reasons, after intervals of 228, 35, and 70 days (for Hospitals A, B, and C), the sampling was repeated. The same materials were used and the same methodology was followed. The same lab performed all testing. The intervals were counted from the day of the first unit installation. Room availability dictated the speed of installation and post-installation testing.
RESULTS
It is well established that UV-C is effective at treating the air. Therefore, results showing that airborne bacteria counts would be lower at the room level post-installation were expected. Because the air would be cleaner after UV-C treatment, we also anticipated a reduction in bacteria on surfaces, which we found in most cases.
By trialing continuous UV-C air purification technology in geographically distinct areas we hoped to discover its efficacy when implemented at the room level. In every case, the amount of airborne bacteria was greatly reduced, and in most cases, surface bacteria was also reduced.
Hospitals reported that healthcare-associated odors were diminished considerably. This was especially evident at Hospital C, where foul odors had been a constant in the closed unit psychiatric holding area, but also mentioned at Hospital A. We believe that cleaning the air with active UV-C technology not only reduced the number of CFUs present, but also resolved odors. This may be due, at least in part, to the UV-C acting on the biological nature of the odors.
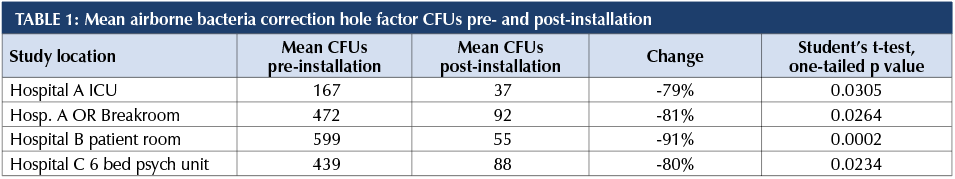
All facilities had significant reductions in airborne bacteria with 24/7 operation of the shielded UV-C ceiling unit.
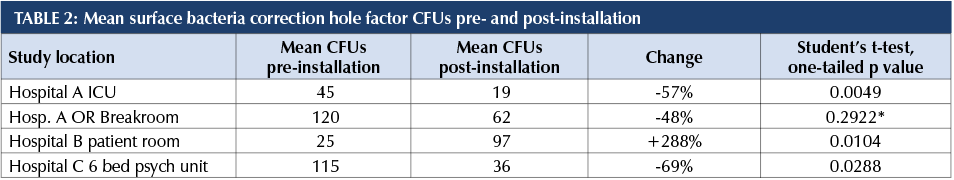
*This p value due to small sample size
Most facilities had significant reductions in surface bacteria after implementing UV-C at the room level.
DISCUSSION
Hospodsky et al. documented human occupancy as the major source of indoor airborne bacteria but observed that the skin, nasal and hair that is shed becomes not only airborne but also settles on surfaces (14). Huang et al. explored the likelihood that a hospital patient could acquire antibiotic-resistant bacteria from someone who had been in the room before (15). Mitchell et al. expanded by performing a meta-analysis on the same topic. They noted the use of UV-C lighting fixtures as a way to reduce the likelihood of a future patient acquiring infection from a prior room occupant (16). King et al. studied surface contamination as a result of airborne disposition of bacteria. They found that small particle bioaerosols are spread with no correlation between surface area of contaminants and distance from the source (17).
Schabrun and Chipchase identified healthcare equipment as a significant source of nosocomial infections (18). Otter et al. agreed that contaminated surfaces are implicated in transmission of pathogens, and further called out UV-C as a disinfection technique with improved efficacy over conventional methods (19). Dumford identified portable hospital equipment as holding reservoirs of C. diff. (20) and Stiefel et al. investigated surfaces as a source of MRSA contamination (21). Shiomori et al. demonstrated that making the bed of a patient with MRSA dispersed MRSA into the air in significant amounts for at least fifteen minutes (22).
Kramer, Schwebke and Kampf looked at how long pathogens can survive on surfaces (23). Acinetobacter spp. survived up to five months, C. diff. up to five months, Escherichia coli up to 16 months, and Staphylococcus aureus, including MRSA, up to seven months. Jawad et al. pointed out that the relative humidity in a space impacts the survival of Acinetobacter spp. and concluded that the bacteria can be transferred from surfaces not only by moist vectors but also by dry ones (24).
It is clear from the literature that bacteria in the air and on surfaces poses a risk to patients, visitors, and staff. Our study showed that using UV-C at the room level reduced the bio burden of the air, and, in most cases, of that on surfaces.
In our study, Hospital B had a very large percentage increase (+288%) in surface bacteria post-installation although the actual numbers weren’t extreme (25, 97). We attribute this to the fact that the study room had been terminally cleaned before pre-installation samples were taken. The pre-installation samples were taken in a cleaned, unoccupied and closed room. The post-installation samples were taken in the room after a patient on isolation had occupied the room and had not been terminally cleaned at the time post-installation samples were taken. This result demonstrates the importance and efficacy of surface cleaning as part of the entire infection control process.
Limitations: A limitation of this study is the location of study sites in fully functioning operational facilities. We had no control over people opening and closing doors thereby affecting airflow into and out of the room, how and how often surfaces were cleaned, as well as the consistent cleaning procedures and the number and types of patients who occupied the spaces. Room furnishings were not identical, nor were layouts. Most patient rooms tested were occupied by patients or work areas were functioning as intended and in use by staff. These variables may have affected the results. A second limitation is the decision to study total bacteria CFUs, and not specific pathogens, fungi, or viruses. Because the study was in live environments, and not in a lab, we had no control over the number and types of pathogens that might be present. Because the hospital environment is dynamic and we did not seed the environment with any given pathogen we used total bacteria load as a surrogate for all pathogens. This approach might be seen as similar to using a biological indicator in a steam sterilizer to ensure hospital equipment and supplies are properly sterilized for use on patients. When the equipment is cleaned and wrapped and sterilized it would be hard to test every potential pathogen, but an indicator helps provide a level of assurance that the equipment is ready for use.
CONCLUSIONS
The data clearly demonstrate that using active air UV-C technology at the room level reduces the bioburden in the air and improves indoor air quality. In addition, the majority of the facilities had reduced surface bacteria in areas where continuous UV-C air purification at the room level was operational. Hospitals should consider adding active air UV-C technology at the room level to decrease airborne and surface microorganisms and improve indoor air quality.
REFERENCES
1. Downes, A. & Blunt, T. P. (1877). Researches on the effect of light upon bacteria and other organisms. Retrieved from https://archive.org/details/philtrans06219880. doi:10.1098/rspl.1877.0068.
2. Coblentz, W. W. & Fulton, H. R. (1924). A radiometric investigation of the germicidal action of ultra-violet radiation. Retrieved from https://archive.org/details/scientificpapers49519geor.
3. Sharp, G. (1939). The lethal action of short ultraviolet rays on several common pathogenic bacteria. Journal of Bacteriology 37, 447-459.
4. Roberts, K., Smith, C. F., Snelling, A. M., Kerr, K. G., Banfield K. R., Sleigh, P. A., & Beggs, C. B. (2008). Aerial Dissemination of Clostridium difficile spores. BMC Infectious Diseases 8 (7) doi:10.1186/1471-2334-8-7.
5. Best, E. L., Fawley, W. N., Parnell, P, & Wilcox, M. H. (2010). The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clinical Infectious Diseases 50 (11), 1450-1457. doi:10.1086/652648.
6. Li, Y., Leung, G. M., Tang, J. W., Yang, X., Chao, C. Y., Lin, J. Z., & Yuen, P. L. (2007). Role of ventilation in airborne transmission of infectious agents in the built environment – a multidisciplinary systematic review. Indoor Air 17 (1) 2-18.
7. Eames, I., Tang, J. W., Li, Y. & Wilson, P. (2009) Airborne transmission of disease in hospitals. Journal of the Royal Society Interface 6 Suppl 6. S698-702. doi:10.1098/rsif.2009.0407.focus.
8. Nazaroff, W. (2014). Indoor bioaerosol dynamics. Indoor Air 26: 61-78. doi:10.1111/ina.12174.
9. Bolton, J. & Cotton, C. (2008). The ultraviolet disinfection handbook. American Waterworks Association.
10. Boyce, J. M. (2016). Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrobial Resistance and Infection Control 5:10 doi:10.1186/s13756-016-0111-x.
11. Rutala, W. A., Gergen, M. F., & Weber, D. J. (2010). Room decontamination with UV radiation. Infection Control & Hospital Epidemiology 31 (10), 1025-1029. doi:10.1086/656244.
12. Reed, N. (2010). The history of ultraviolet germicidal irradiation for air disinfection. Public Health Reports 125 (1) 15-27.
13. Memarzadeh, F., Olmsted, R. N., & Bartley, J. M. (2010). Applications of ultraviolet germicidal irradiation disinfection in health care facilities: effective adjunct, but not stand-alone technology. 38 (Suppl.) 13-24. doi:10.1016/j.ajic.2010.04.208.
14. Hospodsky, D., Qian, J., Nazaroff, W. W., Yamamoto, N., Bibby, K., Rismani-Yazdi, H. & Peccia, J. (2012). Human occupancy as a source of indoor airborne bacteria. PLoS One (7) 4 doi:10.1371/journal.popne.0034867.
15. Huang, S. S., Datta, R., & Platt, R. (2006). Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Archives of Internal Medicine 166 (18), 1945-1951. doi:10.1001/archinte.166.18.1945.
16. Mitchell, B. G., Dancer, S. J., Anderson, M. & Dehn, E. (2015). Risk of organism acquisition from prior room occupants: a systematic review and meta-analysis. Journal of Hospital Infection 91 (3) 211-217. doi:10.1016/j.jhin.2015.08.005.
17. King, M.-F., Noakes, C. J., Sleigh, P. A., & Carmago-Valero, M. A. (2013). Bioaerosol deposition in single and two-bed hospital rooms: a numerical and experimental study. Building and Environment 59 436-447. doi.org/10.1016/j.buildenv.2012.09.011.
18. Schabrun, S. & Chipchase, L. (2006). Healthcare equipment as a source of nosocomial infection: a systematic review. Journal of Hospital Infection, 63 (3), 239-245. doi:10.1016/j.jhin.2005.10.013.
19. Otter, J. A., Yezli, S., Salkeld, J. A., & French, G. L. (2013). Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. American Journal of Infection Control 41 (5 Suppl.), S6-S11. doi:10.1016/j.ajic.2012.12.004.
20. Dumford III, D. M., Nerandzic, M. M., Exkstein, B. C., & Donskey, C. J. (2009). What is on that keyboard? Detecting hidden reservoirs of Clostridium difficile during an outbreak associated with North American pulsed-field gel electrophoresis type 1 strains. American Journal of Infection Control, 37 (1), 15-19. doi:10.1016.j.ajic.2008.07.009.
21. Stiefel, U., Cadnum, J. L., Eckstein, B. C., Guerrero, D. M., Tima, M. A., & Donskey, C. J. (2011). Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infection Control Hospital Epidemiology 32 (2) 185-187. doi:10.1086/657944.
22. Shimori, T., Miyamoto, H., Makishima, K., Yoshida, M., Fujiyoshi, T., Ukada, T., & Hiraki, N. (2002). Evaluation of bedmaking-related airborne and surface methicillin-resistant Staphylococcus aureus contamination. Journal of Hospital Infection, 50 (1), 30-35. doi:10.1053/jhin.2001.1136.
23. Kramer, A., Schwebke, I., & Kampf, G. (2006). How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 6 (130). doi:10.1186/1471-2334-6-130.
24. Jawad, A., Heritage, J., Snelling, A. M., Gascoyne-Binzi, D. M., & Hawkey, P. M. (1996). Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. Journal of Clinical Microbiology, 34 (12), 2281-2287.



