Reprinted with permission from University of Toronto Press (https://utpjournals.press), DOI: 10.3138/jammi-2021-0012; Official Journal of the Association of Medical Microbiology and Infectious Disease Canada.
Robert M Taylor PhD1, James A Karlowsky PhD1,2, Melanie R Baxter MSc1, Heather J Adam PhD1,2, Andrew Walkty MD1,2, Philippe Lagacé-Wiens MD1,2 , George G Zhanel PharmD, PhD1
1Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada;
2Shared Health Manitoba, Winnipeg, Manitoba, Canada
Corresponding author:
George G Zhanel
Department of Clinical Microbiology
Health Sciences Centre Winnipeg
MS673-820
Sherbrook Street
Winnipeg, Manitoba R3A 1R9
Canada
Tel: 204-787-4902
Fax: 204-787-4699
E-mail: ggzhanel@pcsinternet.ca
ABSTRACT
Background: Community-acquired pneumonia (CAP) is a significant global health concern. Pathogens causing CAP demonstrate increasing resistance to commonly prescribed empiric treatments. Resistance in Streptococcus pneumoniae, the most prevalent bacterial cause of CAP, has been increasing worldwide, highlighting the need for improved antibacterial agents. Lefamulin, a novel pleuromutilin, is a recently approved therapeutic agent highly active against many lower respiratory tract pathogens. However, to date minimal data are available to describe the in vitro activity of lefamulin against bacterial isolates associated with CAP.
Methods: Common bacterial causes of CAP obtained from both lower respiratory and blood specimen isolates cultured by hospital laboratories across Canada were submitted to the annual CANWARD study’s coordinating laboratory in Winnipeg, Canada, from January 2015 to October 2018. A total of 876 bacterial isolates were tested against lefamulin and comparator agents using the Clinical and Laboratory Standards Institute (CLSI) reference broth microdilution method, and minimum inhibitory concentrations (MICs) were interpreted using accepted breakpoints.
Results: All S. pneumoniae isolates tested from both respiratory (n = 315) and blood speci¬mens (n = 167) were susceptible to lefamulin (MIC ≤0.5 µg/mL), including isolates resistant to penicillins, clarithromycin, doxycycline, and trimethoprim–sulfamethoxazole. Lefamulin also inhibited 99.0% of Haemophilus influenzae isolates (regardless of β-lactamase production) (99 specimens; MIC ≤2 µg/mL) and 95.7% of methicillin-susceptible Staphylococcus aureus (MSSA) (MIC ≤0.25 µg/mL; 70 specimens) at their susceptible breakpoints.
Conclusions: Lefamulin demonstrated potent in vitro activity against all respiratory isolates tested and may represent a significant advancement in empiric treatment options for CAP.
KEYWORDS
community-acquired pneumonia, Haemophilus influenzae, lefamulin, pleuromutilin, Staphylococcus aureus, Streptococcus pneumoniae
ABSTRAT
Historique : La pneumonie communautaire est une préoccupation sanitaire importante dans le monde. Les agents pathogènes qui en sont responsables démontrent
une résistance croissante envers des traitements empiriques souvent prescrits. La résistance du Streptococcus pneumoniae, la principale cause bactérienne de la pneumonie communautaire, augmente au Canada et dans le monde, ce qui fait ressortir l’importance d’agents antibactériens nouveaux et améliorés. La léfamuline,
une nouvelle pleuromutiline, est un agent thérapeutique récemment homologué qui est très actif contre de nombreux agents pathogènes des voies respiratoires inférieures. Jusqu’à maintenant, peu de données sont toutefois disponibles pour décrire l’activité in vitro de la léfamuline contre les isolats bactériens associés à la pneumonie communautaire.
Méthodologie : Les causes bactériennes courantes de la pneumonie communautaire déterminées à partir d’isolats des voies respiratoires inférieures et d’hémocultures dans des laboratoires canadiens mis en culture par des laboratoires hospitaliers du Canada et soumis à l’étude de surveillance canadienne annuelle dans les services hospitaliers du laboratoire coordonnateur de Winnipeg, au Canada, entre janvier 2015 et octobre 2018. Au total, les chercheurs ont testé 876 isolats bactériens au regard de la lémafuline et des agents comparatifs à l’aide de la méthode de référence de la microdilution dans un milieu de culture du Clinical and Laboratory Standards Institute (CLSI) et ont interprété les concentrations minimales inhibitrices (CMI) d’après les seuils acceptés.
Résultats : La totalité des isolats de S. pneumoniae testés à partir de prélèvements des voies respiratoires (n = 315) et d’hémocultures (n = 167) était susceptible à la léfamuline (CMI ≤0,5 µg/mL), y compris les isolats résistants aux pénicillines, à la clarithromycine, à la doxycycline, au triméthoprime-sulfaméthoxazole et à des isolats multirésistants. La léfamuline inhibait également 99,0 % des isolats d’Haemophilus influenzae (quelle que soit leur production de β-lactamases; n = 99; CMI ≤2 µg/mL) et 95,7 % de ceux de Staphylococcus aureus susceptibles à la méthicilline (SASM; n = 70; CMI ≤0,25 µg/mL) à leurs seuils susceptibles. La léfamuline a dé-montré des valeurs de CMI90 (concentration inhibant 90 % des isolats) de 0,25 µg/mL par rapport au SASM et au S. aureus résistant à la méthicilline (n = 130).
Conclusion : La léfamuline a démontré une puissante activité in vitro au regard de tous les isolats respiratoires testés et peut représenter une avancée importante des traitements empiriques de la pneumonie communautaire.
MOTS-CLÉS
pneumonie communautaire, Haemophilus influenzae, léfamuline, pleuromutiline, Staphylococcus aureus, Streptococcus pneumoniae
BACKGROUND
Community-acquired pneumonia (CAP) is associated with high morbidity, mortality, and economic burden [1-3]. Many pathogens may give rise to CAP, with bacterial infections a prominent cause [1,4]. Streptococcus pneumoniae remains the leading cause of community-acquired bacterial pneumonia (CABP) globally [1-4]. Other bacterial species associated with CABP include Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus, and the intracellular organisms Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila [1].
Treatment of CAP commonly begins with empiric therapy [2,5,6]. First-line agents include macrolides (alone or combined with β-lactams), respiratory fluoroquinolones, and tetracyclines [2,7]. A systematic literature review published in 2017 of studies that investigated S. pneumoniae resistance in the United States reported that between 20% and 40% of isolates were resistant to macrolides [5]. Resistances to clindamycin and trimethoprim–sulfamethoxazole were shown to be approximately 22% and 35%, respectively. Respiratory fluoroquinolone resistance remains low, although fluoroquinolone monotherapy is discouraged because of possible adverse effects and the potential for resistance selection, a commonly observed issue with most antibacterial agents [8,9]. Other studies have also highlighted a growing concern for doxycycline resistance in vitro among bacterial pathogens causing CABP [2,10], which may be correlated with penicillin resistance [5]. Resistance in respiratory isolates of S. pneumoniae has followed a similar trend in Canada, with decreasing susceptibility to penicillin, clarithromycin, doxycycline, and trimethoprim–sulfamethoxazole from 2007 to 2016 [11]. During this time period, the percentage of multi-drug resistance in respiratory isolates of S. pneumoniae (average of 160 specimens yearly) has increased to 9.1% [11].
Even with the introduction of the pneumococcal vaccine, CABP caused by S. pneumoniae and other common respiratory bacterial pathogens remains a prominent health concern, underscoring the need for new and improved antimicrobials to treat this ever-evolving resistant pathogen [5,12]. Several novel antibiotics have been described for treatment of CABP over the past decade, including delafloxacin, omadacycline, nemonoxacin, and solithromycin [13-17]. Currently, none of these are available in Canada or have failed to receive US Food and Drug Administration (FDA) approval because of toxicity risks. An ideal antibiotic for empiric treatment of CABP would possess characteristics that include high clinical efficacy along with minimal adverse reactions and toxicity, a novel mechanism of action (to reduce the potential for cross-resistance with related agents) resulting in activity versus resistant CABP pathogens, activity versus all the most common typical and atypical CABP pathogens, and a high bioavailability that allows for oral (and intravenous) administration.
Lefamulin is a first-in-class, semi-synthetic pleuromutilin available for oral and intravenous administration to treat patients with CABP [3]. The mechanism of action of lefamulin involves inhibition of bacterial protein synthesis through interaction with domain V of the 23S rRNA of the 50S subunit [14]. Lefamulin offers a unique spectrum of activity, is effective as monotherapy treatment for CABP, and is an attractive alternative to both macrolide and fluoroquinolone therapies. Lefamulin has demonstrated potent in vitro activity against pathogens causing CABP, particularly against S. pneumoniae, and it lacks cross-resistance with other antimicrobial classes [18-20].
Lefamulin met predefined noninferiority end points of clinical response for CABP compared with moxifloxacin ± linezolid in two phase III trials (LEAP 1 and 2) [21,22]. Lefamulin (Xenleta™) received FDA approval in 2019 and European Medicines Agency and Health Canada approval in July 2020 for both the intravenous and the oral formulations to treat CABP.
The current study was conducted to assess the in vitro activity of lefamulin against common community-acquired respiratory tract pathogens causing infections in Canada.
Contributors: Conceptualization, MRB, HJA, GGZ; Formal Analysis, RMT, JAK, MRB, HJA, AW, PLW, GGZ; Investigation, JAK, MRB, HJA, GGZ; Data Curation, JAK, MRB, HJA, GGZ; Writing – Original Draft, RMT, JAK, MRB, HJA, GGZ; Writing – Review and Editing, RMT, JAK, MRB, HJA, AW, PLW, GGZ; Supervision, JAK, MRB, HJA, GGZ; Project Administration, MRB; Funding Acquisition, GGZ.
Funding: This work was supported by funding from the Sunnybrook COVID-19 Research Initiative.
Conflicts of interest: The authors have nothing to disclose.
Acknowledgements: The authors thank staff in the following hospital laboratories that participated in the Canadian Ward Surveillance Study: Vancouver Hospital, Vancouver, British Columbia; University of Alberta Hospital, Edmonton, Alberta; Royal University Hospital, Saskatoon, Saskatchewan; Health Sciences Centre, Winnipeg, Manitoba; London Health Sciences Centre, London, Ontario; Hamilton Health Sciences Centre, Hamilton, Ontario; University Health Network/Mount Sinai Hospital, Toronto, Ontario; Children’s Hospital of Eastern Ontario, Ottawa, Ontario; Cité de la Santé de Laval, Laval, Quebec; Jewish General Hospital, Montreal, Quebec; Centre hospitalier régional de Trois-Rivières Pavillon Ste. Marie, Trois-Rivières, Quebec; Centre Hospitalier Universitaire de Sherbrooke, Sherbrooke, Quebec; L’Hôtel-Dieu de Québec, Quebec City, Quebec; South East Regional Health Authority, Moncton, New Brunswick; and Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia.
This work was presented in part at the Association of Medical Microbiology and Infectious Disease Canada–Canadian Association for Clinical Microbiology and Infec¬tious Diseases Annual Conference; April 29-May 2, 2020; Vancouver, British Columbia.
The study included testing against penicillin-resistant, clarithromycin-resistant, doxycycline-resistant, trimethoprim-sulfamethoxazole-resistant, and multidrug-resistant (MDR) S. pneumoniae as well as β-lactamase-positive H. influenzae, methicillin-susceptible S. aureus (MSSA), and methicillin-resistant S. aureus (MRSA).
METHODS
Bacterial isolates
A total of 876 bacterial isolates were tested in the current study. Isolates chosen represented common bacteria associated with CABP. Atypical pathogens known to cause CABP, including M. pneumoniae, C. pneumoniae, and L. pneumophila, were not collected or tested. Isolates were collected as part of the annual CANWARD Surveillance Study from January 2015 to October 2018 by 15 sentinel hospital sites across 8 of the 10 provinces in Canada [23]. CANWARD is an ongoing, national Canadian Antimicrobial Resistance Alliance-Health Canada partnered study assessing antimicrobial resistance patterns of pathogens causing infections among patients receiving care at hospitals across Canada. Isolates were submitted to the CANWARD Surveillance Study coordinating laboratory (Winnipeg Health Sciences Centre, Winnipeg, Manitoba, Canada). Each sentinel hospital site was asked to collect and submit 100 consecutive lower respiratory tract infection specimen pathogens per year as deemed significant by their site. Laboratories collected isolates non-selectively to obtain a representative sample of organisms recovered from specific infection sites by each laboratory during routine diagnostic work. Isolates were limited to one per patient, and both inpatient and outpatient isolates were accepted. Specimen sources included sputum, tracheal aspirate, bronchoalveolar lavage, and bronchoscopy wash-protective brush specimens. From this sample of lower respiratory tract infection pathogens (CANWARD January 2015-October 2018 inclusive), all S. pneumoniae, H. influenzae, M. catarrhalis, and S. aureus were selected for testing. In addition, all bacteremic isolates of S. pneumoniae collected from CANWARD from January 2015-October 2018 were also included. Species identities were confirmed biochemically or by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (Bruker Daltonics; Billerica, Massachusetts, USA).
Antimicrobial susceptibility testing
Minimum inhibitory concentrations (MICs] for lefamulin and comparator agents were determined using the Clinical and Laboratory Standards Institute (CLSI] reference broth microdilution method [24,25] with 96-well custom-designed microtitre plates containing doubling dilutions of agents in volumes of 100 µL/well. Quality control testing was performed each day on which clinical isolates were tested, as specified by CLSI [24,25]. Colony counts were performed periodically to confirm starting inocula. Lefamulin MICs were interpreted using FDA interpretive criteria [26]: S. pneumoniae, ≤0.5 µg/mL susceptible; H. influenzae, ≤2 µg/mL susceptible; and MSSA, ≤0.25 µg/mL susceptible. Ceftobiprole MICs were interpreted using interpretive criteria obtained from the PrZEVTERA® product monograph [27]. MICs to other agents for S. pneumoniae, H. influenzae, and S. aureus were interpreted using 2020 CLSI M100 criteria [24]. Oral penicillin breakpoints were used to determine sensitive (≤0.06 µg/mL), intermediate (0.12–1 µg/mL), and resistant
(≥2 µg/mL) isolates. MICs for agents tested against M. catarrhalis were interpreted using 2015 CLSI M45 criteria [28]. β-lactamase was tested for H influenzae using a nitrocefin colourmetric assay [29]. Methicillin susceptibility in S. aureus isolates was determined according to CLSI criteria [24].
RESULTS
The 876 bacterial isolates (and their graded percentage) that were identified and analyzed in this study included S. pneumoniae (55.0%), S. aureus (22.8%), H. influenzae (11.3%), and M. catarrhalis (10.9%).
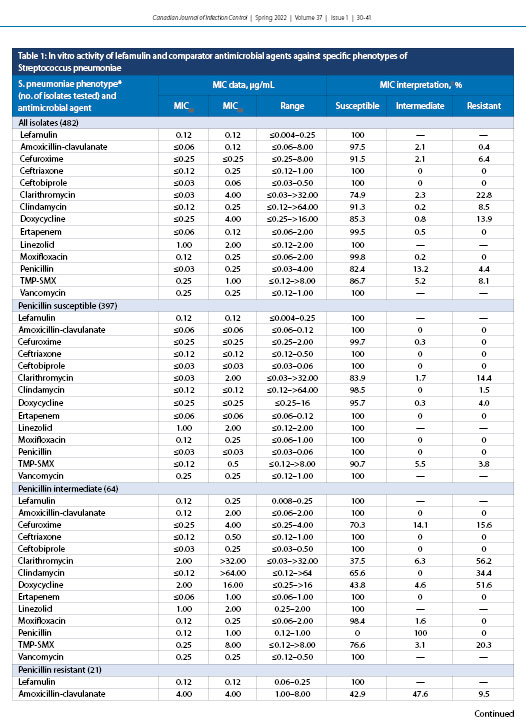
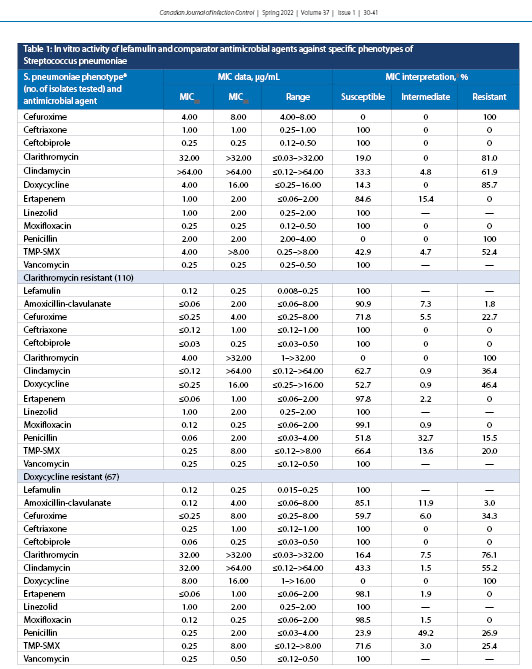
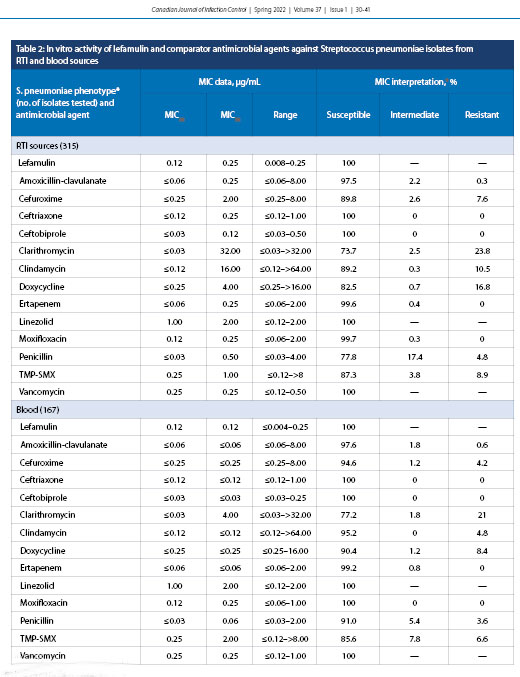
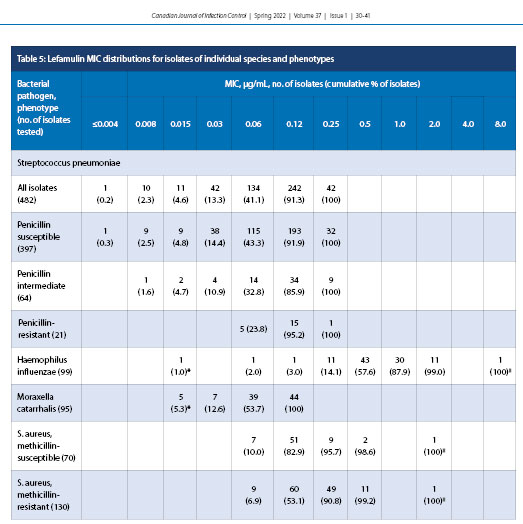
The concentration of lefamulin inhibiting 50% (MIC50) and 90% (MIC90) of all S. pneumoniae isolates (n = 482) were 0.12 and 0.12 µg/mL, respectively (Table 1). Lefamulin MICs ranged from ≤0.004 to 0.25 µg/mL, and all isolates tested as susceptible to lefamulin. Lefamulin was also shown to have a MIC50 of 0.12 and MIC90 of 0.12 µg/mL against penicillin-susceptible (397) and penicillin-resistant (21) S. pneumoniae. The penicillin-intermediate (64), clarithromycin-resistant (110), and doxycycline-resistant (67) isolates had MIC90 values elevated by one doubling dilution to 0.25 µg/mL. It should be noted that higher-level penicillin resistance was not evaluated because even these penicillin-resistant isolates displayed 100% sensitivity to ceftriaxone and ceftobiprole. Trimethoprim-sulfamethoxazole-resistant (MIC ≥4.0 µg/mL) (39; data not shown) and MDR (resistant to ≥3 antimicrobial classes) [10] isolates were also analyzed against S. pneumoniae, displaying 100% susceptibility to lefamulin. Lefamulin was equally active against bacteremic (167) and respiratory (315) isolates of S. pneumoniae, with identical MIC50 values of 0.12 µg/mL (Table 2).
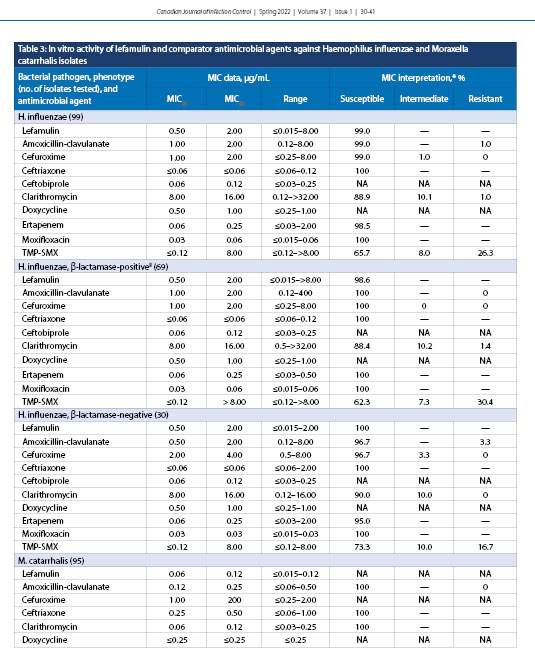
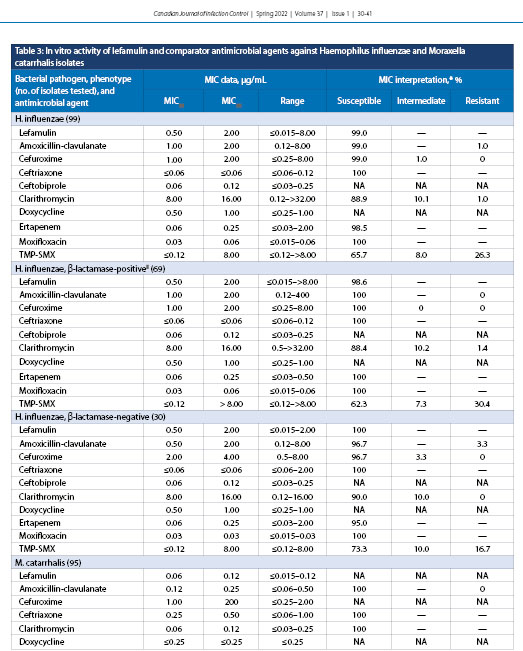
Lefamulin demonstrated MIC50 and MIC90 of 0.5 and 2.0 µg/mL, respectively, against all H. influenzae (99), β-lactamase-positive (69) isolates, and β-lactamase-negative isolates [30], with MICs ranging from ≤0.015 to >8.0 µg/mL; 99.0% of all isolates were susceptible to lefamulin (Table 3). Lefamulin demonstrated MIC50 and MIC90 of 0.06 and 0.12 µg/mL, respectively, for M. catarrhalis (95) with an MIC range of ≤0.015–0.12 µg/mL.
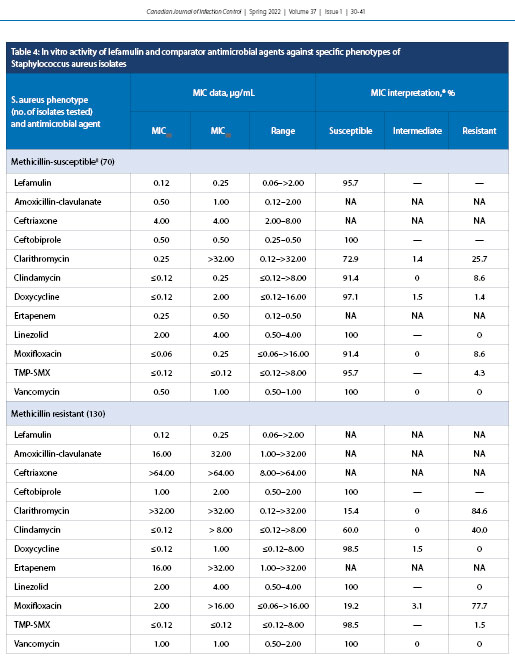
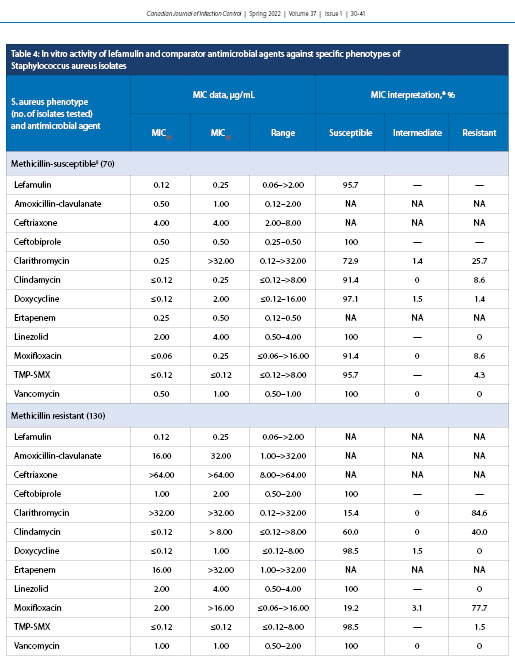
Lefamulin exhibited MIC50 and MIC90 of 0.12 and 0.25 µg/mL, respectively, against MSSA (70 specimens) with a MIC range of 0.06 to >2.0 µg/mL with 95.7% susceptibility (Table 4). Lefamulin also had MIC50 and MIC90 values of 0.12 and 0.25 µg/mL, respectively, against MRSA (130 specimens) with an MIC range of 0.06 to >2.0 µg/mL (percent susceptibility is unknown because FDA breakpoints for lefamulin do not include MRSA).
Note: Dashes indicate no data available.
* Penicillin susceptible was defined as MIC ≤0.06 μg/mL, penicillin intermediate as MIC 0.12–1 μg/mL, penicillin resistant as MIC ≥2 μg/mL, clarithromycin resistant as MIC ≥1 μg/mL, and doxycycline-resistant as MIC ≥1 μg/mL.
† Percent susceptibility was determined according to Clinical and Laboratory Standards Institute 2020 breakpoints, with the exceptions of those for lefamulin, for which US Food and Drug Administration breakpoints were applied, and ceftobiprole, for which Health Canada-approved breakpoints were used. MIC = Minimum inhibitory concentration; TMP-SMX = Trimethoprim-sulfamethoxazole
Note: Dashes indicate no data available.
* Percent susceptibility was determined according to Clinical and Laboratory Standards Institute 2020 breakpoints, with the exceptions of those for lefamulin, for which US Food and Drug Administration breakpoints were applied, and ceftobiprole, for which Health Canada–approved breakpoints were used.
RTI = Respiratory tract infection; MIC = Minimum inhibitory concentration; TMP–SMX = Trimethoprim–sulfamethoxazole
Note: Dashes indicate no data available.
* Percent susceptibility was determined according to CLSI 2020 breakpoints, with the exceptions of those for lefamulin, for which U.S. Food and Drug Administration
breakpoints were applied; ceftobiprole, for which Health Canada-approved breakpoints were used; and antimicrobial agents for M. catarrhalis, for which CLSI M45
2015 breakpoints were applied.
†β-lactamase production for H. influenzae was analyzed according to the 2016 Clinical Microbiology Procedures Handbook.
MIC = Minimum inhibitory concentration; NA = Not applicable (there are no MIC breakpoints defined for this antimicrobial agent or there were <30 isolates tested and an
MIC50 and MIC90 could not be generated); TMP–SMX = Trimethoprim–sulfamethoxazole; CLSI = Clinical and Laboratory Standards Institute
Note: Dashes indicate no data available
* Percent susceptibility was determined according to CLSI 2020 breakpoints, with the exceptions of those for lefamulin, for which US Food and Drug Administration breakpoints were applied, and ceftobiprole, for which Health Canada-approved breakpoints were used
† Methicillin susceptibility for S. aureus isolates was tested according to 2020 CLSI standards
MIC = Minimum inhibitory concentration; CLSI = Clinical and Laboratory Standards Institute; NA = Not applicable (there are no MIC breakpoints defined for this antimicrobial agent or there were <30 isolates tested and an MIC50 and MIC90 could not be generated); TMP–SMX = Trimethoprim–sulfamethoxazole
Note: Dashes indicate no data available.
* Percent susceptibility was determined according to CLSI 2020 breakpoints, with the exceptions of those for lefamulin, for which U.S. Food and Drug Administration
breakpoints were applied; ceftobiprole, for which Health Canada-approved breakpoints were used; and antimicrobial agents for M. catarrhalis, for which CLSI M45
2015 breakpoints were applied.
†β-lactamase production for H. influenzae was analyzed according to the 2016 Clinical Microbiology Procedures Handbook.
MIC = Minimum inhibitory concentration; NA = Not applicable (there are no MIC breakpoints defined for this antimicrobial agent or there were <30 isolates tested and an
MIC50 and MIC90 could not be generated); TMP–SMX = Trimethoprim–sulfamethoxazole; CLSI = Clinical and Laboratory Standards Institute
Note: Dashes indicate no data available
* Percent susceptibility was determined according to CLSI 2020 breakpoints, with the exceptions of those for lefamulin, for which US Food and Drug Administration breakpoints were applied, and ceftobiprole, for which Health Canada-approved breakpoints were used
† Methicillin susceptibility for S. aureus isolates was tested according to 2020 CLSI standards
MIC = Minimum inhibitory concentration; CLSI = Clinical and Laboratory Standards Institute; NA = Not applicable (there are no MIC breakpoints defined for this antimicrobial agent or there were <30 isolates tested and an MIC50 and MIC90 could not be generated); TMP–SMX = Trimethoprim–sulfamethoxazole
DISCUSSION
The current study confirmed that lefamulin, a novel pleuromutilin, is as active as or more active than (based on percent susceptible rates) amoxicillin-clavulanate, cefuroxime, ceftriaxone, ceftobiprole, clarithromycin, clindamycin, doxycycline, ertapenem, moxifloxacin, penicillin, and trimethoprim–sulfamethoxazole in vitro against common bacterial pathogens associated with CABP obtained from across Canada by the CANWARD surveillance study from January 2015 to October 2018. Increasing resistance to β-lactams and other first-line empiric antibacterial agents among pathogens causing CABP, particularly in S. pneumoniae because it is responsible for the majority of cases, is a growing concern worldwide [5,14,21,22]. Lefamulin was shown in the current study to retain its potency in vitro against both penicillin-susceptible and penicillin-resistant phenotypes of S. pneumoniae, with MIC50 and MIC90 values ranging from 0.12 to 0.25 µg/mL. Three other in vitro studies have each reported analogous values for MIC50 and MIC90 of 0.06 and 0.12 µg/mL, respectively [30-33]. The susceptibility of S. pneumoniae isolates to clarithromycin correlates directly with penicillin resistance, with an MIC50 value of ≤0.03 µg/mL for penicillin-susceptible isolates compared with 32.0 µg/mL for penicillin-resistant isolates.
The same trend was found for doxycycline, with MIC50 values varying from ≤0.25 µg/mL (penicillin-susceptible) to 4.0 µg/mL (penicillin-resistant) against isolates of S. pneumoniae. Lefamulin potency was also retained when tested against S. pneumoniae isolates resistant to clarithromycin, doxycycline, and trimethoprim–sulfamethoxazole. Overall, at a MIC value of ≤0.5 µg/mL, the FDA-approved breakpoint, all phenotypes of S. pneumoniae were shown to be 100% susceptible to lefamulin (Table 5).
H. influenzae, another well-established cause of CABP, was shown in the LEAP 1 clinical trial to account for 34% of all respiratory pathogens in patients with a baseline pathogen detected [21]. In this study, lefamulin demonstrated MIC50 and MIC90 values of 0.5 µg/mL and 2.0 µg/mL, respectively, against H. influenzae (Table 3). A similar in vitro study in 2019 reported corresponding data, with MIC50 and MIC90 values of 0.5 µg/mL and 1.0 µg/mL, respectively [32]. β-lactamase-positive isolates of H. influenzae demonstrated an MIC range that was slightly elevated for β-lactamase-negative isolates (≤0.015-2.0 µg/mL) compared with β-lactamase-positive isolates (≤0.015-8.0 µg/mL). Nevertheless, 99% of all H. influenzae isolates were susceptible to lefamulin (Table 5). Clarithromycin again showed a dependence on penicillin susceptibility, with β-lactamase-positive isolates of H. influenzae having an elevated MIC range. M. catarrhalis was shown to have low MIC50 and MIC90 values for lefamulin at 0.06 µg/mL and 0.12 µg/mL, respectively (Table 3). These values are identical to those from a similar study in 2018 that tested 667 M. catarrhalis isolates obtained globally from the SENTRY Antimicrobial Surveillance Program 2015-2016 [32].
The in vitro activity of lefamulin was additionally evaluated against another cause of CABP, S. aureus (Table 4). Lefamulin had 95.7% susceptibility toward MSSA, with MIC50 and MIC90 values of 0.12 and 0.25 µg/mL, respectively. MIC breakpoints do not currently exist for MRSA, but the MIC values generated in the current study for MRSA were identical to those for MSSA. Resistance has rarely been observed for lefamulin among its target pathogens; mechanisms of resistance to lefamulin have been shown to be spontaneous and to be related to modification of 23S rRNA ribosomal target proteins [34-36]. Most commonly, the genes rplC and rplD have been shown to facilitate single-point mutations in the ribosomal proteins, leading to a conformational change that hinders the ability of lefamulin to properly bind [34]. If patients require a longer duration of hospital admission, nosocomial bacteria including Klebsiella pneumoniae and Pseudomonas aeruginosa may lead to more severe outcomes. Lefamulin has been shown to be relatively inactive against these bacterial species, similar to what is seen for other first-line CABP antibacterial agents [6,14]. Nevertheless, two other studies have reported results similar to ours, with MIC50 and MIC90 values of 0.06 and 0.12 µg/mL, respectively [31,32]. In contrast, moxifloxacin, a common first-line fluoroquinolone used in CABP treatment, has been shown to demonstrate a marked MIC90 increase, with a reduction in susceptibility from 91.4% (MSSA) to 19.2% (MRSA). Lefamulin exhibited an MIC90 (0.25 µg/mL) against MSSA that was 128 times more potent than clarithromycin and eight times more potent than doxycycline. Interestingly, doxycycline exhibited minimal variation in susceptibility between MSSA and MRSA. Thus, it has been speculated that a role in empiric treatment of CABP due to S. aureus could involve an approach that involves a combination of both doxycycline and lefamulin, with in vitro data suggesting a potential for synergy [35,37].
This current study also outlined the in vitro activity of lefamulin against possible systemic infection with S. pneumoniae, because respiratory tract infections have been well characterized as possibly increasing the risk of bacteremia [37]. Potency against S. pneumoniae isolates from both respiratory tract infections and blood sources was maintained (Table 2), suggesting potential activity of lefamulin for severe and systemic downstream effects of CABP. Of note is the fact that most other antimicrobials tested displayed general increased susceptibility for blood isolates (Table 2). This can be partly attributed to the resistance patterns seen across Canadian hospitals, which was outlined in a 2019 CANWARD report [11]. Since the introduction of the pneumococcal conjugate vaccine between 2002 and 2005, the serotypes commonly associated with penicillin resistance have decreased [11]. However, serotype 19A, a significant source of respiratory disease in Canada, has been shown to be associated with higher penicillin resistance [11].
CONCLUSION
The intent of this work was to add to the limited in vitro data regarding the potency of lefamulin against common bacterial causes of CABP. With bacterial resistance to S. pneumoniae on the rise, the need for new and improved antibiotics is paramount. Lefamulin, a novel pleuromutilin, exhibited excellent in vitro activity against all S. pneumoniae isolates (blood or respiratory origin) tested with 100% of isolates susceptible to lefamulin (MIC values ≤0.5 µg/mL), including isolates of S. pneumoniae resistant to penicillins, cephalosporins, clarithromycin, doxycycline, and trimethoprim-sulfamethoxazole, as well as MDR isolates. In addition, 99% of H. influenzae isolates were susceptible to lefamulin, as were 95.7% of MSSA. Both MSSA and MRSA tested with an MIC90 value of 0.25 µg/mL for lefamulin. The approval of lefamulin by Health Canada for oral and intravenous use represents an advance in empiric treatment options for patients with CABP
in Canada.
* Lowest concentration tested, actual MIC may be lower
† Highest concentration tested, actual MIC may be higher MIC = Minimum inhibitory concentration
REFERENCES
1. Peyrani P, Mandell L, Torres A, Tillotson GS. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13(2):139–52. https://doi.org/10.1080/17476348.2019.1562339. Medline:30596308
2. Mandell LA. Something new for community-acquired pneumonia? Clin Infect Dis. 2016;63(12):1681–2. https://doi.org/10.1093/cid/ciw609. Medline:27789608
3. Dillon C, Guarascio AJ, Covvey JR. Lefamulin: a promising new pleuromutilin antibiotic in the pipeline. Expert Rev Anti Infect Ther. 2019;17(1):5–15. https://doi.org/10.1080/14787210.2019.1554431. Medline:30513017
4. Ho J, Ip M. Antibiotic-resistant community-acquired bacterial pneumonia. Infect Dis Clin North Am. 2019;33(4):1087–103. https://doi.org/10.1016/j.idc.2019.07.002. Medline:31668192
5. Cherazard R, Epsetin M, Doan T, Salim T, Bhart S, Smith MA. Antimicrobial resistant Streptococcus pneumoniae: prevalence, mechanisms, and clinical implications. Am J Ther. 2017;24(3):361–369. https://doi.org/10.1097/MJT.0000000000000551. Medline:28430673
6. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia—an official clinical practice guideline for the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581ST. Medline:31573350
7. Sharma R, Sandrock CE, Meehan J, Theriault N. Community-acquired bacterial pneumonia—changing epidemiology, resistance patterns, and newer antibiotics: spotlight on delafloxacin. Clin Drug Investig. 2020;40(10):947–960. https://doi.org/10.1007/s40261-020-00953-z. Medline:32889706
8. Frei CR, Labreche MJ, Attridge RT. Fluoroquinolones in community-acquired pneumonia. Drugs. 2011;71(6):757–70. https://doi.org/10.2165/11585430-000000000-00000. Medline:21504252
9. Aschenbrenner D. The FDA revises boxed warning for fluoroquinolones—again. Am J Nurs. 2016;116(9):22–3. https://doi.org/10.1097/01.NAJ.0000494691.55746.90. Medline:27560334
10. Bushra R, Rizvi M, Shafiq Y, Khan M, Zafar F, Ali H. Resistance emergence of doxycycline against Staphylococcus aureus and Streptococcus pneumoniae. Pak Armed Forces Med J. 2020;70(Supp 1):S188–92. https://www.pafmj.org/index.php/PAFMJ/article/view/3990
11. Golden AR, Baxter MR, Davidson RJ, et al. Comparison of antimicrobial resistance patterns in Streptococcus pneumoniae from respiratory and blood cultures in Canadian hospitals from 2007-16. J Antimicrob Chemother. 2019;74(Supp 4):iv39–47. https://doi.org/10.1093/jac/dkz286. Medline:31505644
12. Karlowsky JA, Adam HJ, Golden AR, et al. Antimicrobial susceptibility testing of invasive isolates of Streptococcus pneumoniae from Canadian patients: the SAVE study, 2011-15. J Antimicrob Chemother. 2018;73(Supp 7):vii5-11. https://doi.org/10.1093/jac/dky156. Medline:29982570
13. Zhanel GG, Hartel E, Adam H, et al. Solithromycin: a novel fluoroketolide for the treatment of community-acquired bacterial pneumonia. Drugs. 2016;76(918):1737–57. https://doi.org/10.1007/s40265-016-0667-z. Medline:27909995
14. Kollef MH, Betthauser KD. New antibiotics for community-acquired pneumonia. Curr Opin Infect Dis. 2019;32(2):169–75. https://doi.org/10.1097/QCO.0000000000000526. Medline:30640820
15. Scott LJ. Delafloxacin: a review in acute bacterial skin and skin structure infections. Drugs. 2020;80(12):1247–58. https://doi.org/10.1007/s40265-020-01358-0. Medline:32666425
16. Zhanel GG, Esquivel J, Zelenitsky S, et al. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs. 2020;80(3):285–313. https://doi.org/10.1007/s40265-020-01257-4. Medline:31970713
17. Guo B, Qu X, Zhang Y, et al. Safety and clinical pharmacokinetics of nemonoxacin, a novel non-fluorinated quinolone, in healthy Chinese volunteers following single and multiple oral doses. Clin Drug Investig. 2012;32(7):475–86. https://doi.org/10.2165/11632780-000000000-00000. Medline:22650326
18. Mendes RE, Farrell DJ, Flamm RK, et al. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. J Antimicrob Chemother. 2016;60(7);4407–11. https://doi.org/10.1128/AAC.00627-16. Medline:27161634
19. Sader HS, Paukner S, Ivezic-Schoenfeld Z, Biedenbach DJ, Schmitz FJ, Jones RN. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). Antimicrob Agents Chemother. 2012;67(5):1170–5. https://doi.org/10.1093/jac/dks001. Medline:22287234
20. Waites KB, Crabb DM, Duffy LB, Jensen JS, Liu Y, Paukner S. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother. 2017;61(2):e02008–16. https://doi.org/10.1128/AAC.02008-16. Medline:27855075
21. File TM, Goldberg L, Das AF, et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial. Clin Infect Dis. 2019;69(11):1856–67. https://doi.org/10.1093/cid/ciz090. Medline:30722059
22. Alexander E, Goldberg L, Das AF, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia. The LEAP 2 randomized clinical trial. JAMA. 2019;322(17):1661–71. https://doi.org/10.1001/jama.2019.15468. Medline:31560372
23. Zhanel GG, Adam HJ, Baxter MR, et al. 42936 pathogens from Canadian hospitals: 10 years of results (2007–16) from the CANWARD surveillance study. J Antimicrob Chemother. 2019;74(Supp 4):iv5–21. https://doi.org/10.1093/jac/dkz283. Medline:31505641
24. Clinical and Laboratory Standards Institute (CLSI). M100: performance standards for antimicrobial susceptibility testing. 30th ed. Wayne, PA: CLSI; 2020. https://clsi.org/media/3481/m100ed30sample.pdf (Accessed December 17 2020).
25. Clinical and Laboratory Standards Institute (CLSI). M07-A11: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard. 11th ed. Wayne, PA: CLSI; 2018. https://clsi.org/media/1928/m07ed11sample.pdf (Accessed December 17, 2020).
26. US Food and Drug Administration. Lefamulin oral and injection products. 2019. https://www.fda.gov/drugs/development-resources/lefamulin-oral-and-injection-products (Accessed January 4, 2021).
27. AVIR Pharma Inc. PrZEFTERA®: ceftobiprole medocaril powder for injection. 2018. https://www.avirpharma.com/pdf/Product-Monograph-Zevtera.pdf (Accessed December 17, 2020).
28. Clinical and Laboratory Standards Institute (CLSI). M45: methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria. 3rd ed. Wayne, PA: CLSI; 2015. https://clsi.org/media/1450/m45ed3sample.pdf (Accessed December 17, 2020).
29. Leber AL. Clinical microbiology procedures handbook. 4th ed. Washington, DC: American Society of Microbiology Press; 2016.
30. Bhavani SM, Zhang L, Hammel JP, et al. Pharmacokinetic/pharmacodynamic target attainment analyses to support intravenous and oral lefamulin dose selection for the treatment of patients with community-acquired bacterial pneumonia. J Antimicrob Chemother. 2019;74(Supp 3):iii35–41. https://doi.org/10.1093/jac/dkz089. Medline:30949705
31. Paukner S, Gelone SP, Arends SJR, Flamm RK, Sader HS. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015-2016). Antimicrob Agents Chemother. 2019;63(4):e02161–18. https://doi.org/10.1128/AAC.02161-18. Medline:30670415
32. Mendes RE, Farrell DJ, Flamm RK, et al. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respira-tory tract infections in the United States. Antimicrob Agents Chemother. 2016;60(7):4407–11. https://doi.org/10.1128/AAC.00627-16. Medline:27161634
33. Mercuro NJ, Veeve MP. Clinical utility of lefamulin: if not now, when? Curr Infect Dis Rep. 2020;22(9):22–5. https://doi.org/10.1007/s11908-020-00732-z. Medline:32834786
34. XENLETA™: lefamulin injection, for intravenous use & lefamulin tablets, for oral use. King of Prussia, PA: Nabriva Therapeutics US, Inc.; 2019. https://www.accessdata.fda.gov/drugsatfdadocs/label/2019/211672s000,211673s000lbl.pdf (Accessed December 17, 2020).
35. Mendes RE, Paukner S, Doyle TB, Gelone SP, Flamm RK, Sader HS. Low prevalence of Gram-positive isolates showing elevated lefamulin MIC results during the SENTRY surveillance program for 2015-2016 and characterization of resistance mechanisms. J Antimicrob Chemother. 2019;63(4):e02158–18. https://doi.org/10.1128/AAC.02161-18. Medline:30670415
36. Kozhokar L, Levien TL, Baker DE. Lefamulin. Hospital Pharm. Forthcoming. https://doi.org/10.1177/0018578719897071.
37. Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. AM J Respir Crit. 2003;169(3):342–7. https://doi.org/10.1164/rccm.200309-1248OC. Medline:14630621



