Devon Metcalf, PhD, CIC1* and Bois Marufov, MD, MSc, CIC1
1Public Health Ontario, Toronto, Canada
*Corresponding author:
Devon Metcalf
Public Health Ontario, Canada
Email: devon.metcalf@oahpp.ca
ABSTRACT
Background: Establishing a robust, standardized and validated surveillance system in long-term care (LTC) homes is a necessary strategy to assess and analyze
infection trends over time, inform infection prevention and control (IPAC) practices in order to reduce healthcare-associated infections and be compliant with
legislative requirements.
Methods: To support strong surveillance programs in LTC, a surveillance toolkit was developed and trialed in a LTC corporation consisting of eighteen LTC homes
across Southern Ontario. The tool was developed, piloted and trialed using available best practices and revised based on feedback from the LTC IPAC Leads.
An evaluation was conducted using formal telephone and in-person interviews, online surveys and informal discussions through regular webinars.
Results: Suggestions for improvements to the toolkit included a preference for forms that automated case counting and rate calculations and the removal of tools or sections of tools deemed unnecessary by the user.
Conclusion: Although the IPAC Leads did not use all of the tools consistently, they felt the toolkit improved their surveillance process by increasing the standardization
and consistency of the tracking of infections.
KEYWORDS
Surveillance, long-term care, infection prevention and control
INTRODUCTION
Healthcare-associated infections (HAIs), defined as infections acquired during the provision of care and not present or incubating prior to care are common in long-term care homes (LTCHs). These infections may cause outbreaks which may lead to morbidity or mortality [1]. A robust infection surveillance system, involving data collection, analysis and reporting, can help reduce HAIs by identifying cases early in order to plan timely interventions to reduce or prevent the transmission of infectious agents [2]. Such a system can also improve the understanding of the burden of infections and help assess if interventions are working. Surveillance programs are a key component of an infection prevention and control (IPAC) program and Ontario LTCHs are required to have IPAC programs that involve monitoring infections in residents [3].
Improved support for surveillance programs in LTCHs is currently needed. Following an IPAC needs assessment survey conducted in 2019, LTCHs identified surveillance as the second most common area in need of improvement in their organizations and surveillance was the second most common area that participants required further training in [4]. This study assesses the need for simplified surveillance tools for use in LTC settings. The COVID-19 pandemic has also highlighted the need for robust IPAC practices in LTC settings, including the need for reliable surveillance programs to ensure timely identification of infectious disease agents [5-7]. To the best of our knowledge, tools to support LTCHs in developing, implementing and sustaining a surveillance system have not been freely available. Therefore, the aim of this study was to design a standardized set of infection surveillance tools for use in LTC and to describe the challenges and successes in developing and trialling them.
Methods
Surveillance toolkit development
A set of tools to support infection surveillance in LTC was developed by IPAC specialists from the provincial government agency, Public Health Ontario (PHO). Best practice documents such as those produced by the Provincial Infectious Diseases Advisory Committee were used to inform the development of the tools [1, 8, 9]. Standardized case definitions developed by Stone [10] were used as the cases definitions for this toolkit. The original toolkit consisted of: 1. a daily surveillance form for tracking signs and symptoms of infections, 2. case definitions [10], 3. case validation forms to assist in applying the case definitions, 4. a monthly HAI case tracker, 5. a monthly antimicrobial-resistant organism (ARO) infection case tracker, 6. an annual infection case log, 7. an annual infection rate tracker, 8. methicillin-resistant Staphylococcus aureus annual log, 9. vancomycin-resistant entercocci annual log, 10. staff sick call form, 11. acute respiratory infection (ARI) screening tool, 12. report form to track IPAC issues identified and staff education provided, 13. patient transfer authorization form, 14. an introduction to surveillance document, 15. a sample surveillance policy and procedure.
Pilot of the surveillance toolkit
Two local LTCHs piloted the toolkit from October to December 2017. One LTCH had approximately 100 beds and the other had approximately 225 beds. A convenience sample of two homes was selected for participation in the trial because they had identified a need to improve their infection surveillance system and was able to commit to a short trial of the surveillance tools. Training was provided by PHO IPAC Specialists to the two LTC IPAC Leads who then implemented the toolkit and began to use the tools in the collection and analysis of their infection surveillance data. Monthly check-ins were used to gather feedback and to make improvements and revisions to the tools throughout the trial. Upon completion, an unstructured interview was conducted to gather feedback on the overall process and suggestions for improvement.
Based on feedback, several tools were removed or replaced and the revised toolkit consisted of: 1. a protocol for using the tools, 2. a readiness self-assessment worksheet, 3. a set of training slides, 4. a daily surveillance form used to document signs and symptoms of infections, 5. case definitions [10], 6. case validation forms that assist in applying the case definitions to verify that the residents exhibiting signs and/or symptoms of infections meet the case definition, and 7. a surveillance reporting form that is used to collate monthly data from residents who meet the case definition.
Trial of the revised toolkit
PHO collaborated with an eighteen home LTC corporation located in Southern Ontario to trial the revised toolkit from April 2018 to April 2019. This corporation was selected because of a self-identified need for improvement to their surveillance program and their willingness to collaborate on the trial and revision of the toolkit. Additionally, implementation of the toolkit in a LTC corporation provided the opportunity to assess how well the toolkit worked in LTCHs following the same overarching policies related to surveillance. In addition to the eighteen IPAC Leads, a corporate infection control professional (ICP) and health informatics coordinator (HICo) also supported this initiative. The HICo worked closely with PHO to revise the surveillance reporting form to include features such as automated incidence rate calculations and the generation of graphs, as dictated by user feedback.
An in-person training session led by PHO IPAC Specialists was offered to the IPAC Leads in March 2018 that included presentations and group discussions on the importance of surveillance followed by an interactive demonstration of the tools and completion of the readiness self-assessment. A gradual implementation process occurred starting in April 2018 with one infection-type (i.e., urinary tract infections (UTIs)) for a period of two months. UTIs were chosen to start because of the common frequency of this infection type. Additional infection types (selected at random) were added at regular intervals over the course of the next eight months (i.e., ARIs, then gastrointestinal infections followed by other infections). The HICo collected all surveillance reporting forms monthly and created an aggregate data-driven trend report. Seven webinars were offered semi-monthly to provide opportunities to gather feedback from the IPAC Leads, answer questions, and troubleshoot challenges.
Process evaluation
Feedback on how the tools were performing was gathered from the IPAC Leads (see Appendix), the corporate ICP and the HICo through informal sharing throughout the trial (i.e., during seven check-in webinars intended to provide support throughout the trial) and during a comprehensive final evaluation. This evaluation was performed six months after completion of the implementation. The purpose was to identify ways to improve the tools as well as understanding the barriers and challenges to implementation of the toolkit, which will be discussed in a forthcoming article.
An online survey was developed to evaluate the experiences of the frontline nurses who had used the daily surveillance form. The IPAC Leads were responsible for survey promotion and the survey was kept open for three weeks. Additionally, a structured telephone interview was conducted for the IPAC Leads and a semi-structured in-person interview was conducted for the HICo and the corporate ICP. This format was chosen for the HICo and corporate ICP evaluation to allow for in-depth discussion about their perceptions and experiences. All interviews were conducted by a practicum student in the summer of 2019 and a descriptive analysis of responses was performed. Responses were analyzed and grouped into themes and used to inform improvements to the toolkit.
This project was not submitted for research ethics board approval as the activities described here are outside of the scope of ethics board review as per the Tri-Council Policy Statement 2 (2018), Article 2.5: “Quality assurance and quality improvement studies, program evaluation activities, and performance reviews, or testing within normal educational requirements when used exclusively for assessment, management or improvement purposes, do not constitute research for the purposes of this Policy, and do not fall within the scope of REB review”[11].
Results
Feedback from the pilot
The two IPAC Leads involved in the initial pilot felt that the toolkit strengthened their surveillance program but also provided suggestions for improvement. They identified redundancies in the tools, the need for automation and the need to streamline the process to ensure IPAC Leads have capacity to complete the surveillance in addition to other daily tasks. As a result, revisions occurred prior to implementation in the LTC corporation including the consolidation of the monthly and annual ARO and other infection case trackers into one tool and removal of several tools deemed unnecessary. The patient transfer authorization form, the staff sick call form, the sample policy and the ARI screening tool were removed because the homes indicated that they, and likely other homes had adequate existing forms or processes. Additionally, an assessment tool to ensure readiness and a training webinar was created to support training staff on the importance of surveillance and how to use the tools.
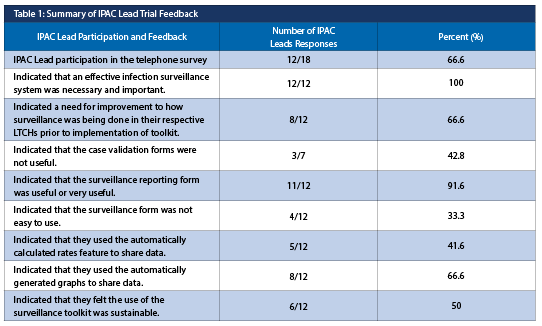
IPAC lead trial feedback
During the check-in webinars, the IPAC Leads wanted further automation of the surveillance reporting form (e.g., to include the generation of graphs and the automatic calculation of rates). Additionally, a formal evaluation was conducted involving a structured telephone interview covering preparedness, implementation, and the opinions about the specific tools (results informing the development of the final version of the toolkit are summarized in Table 1).
When asked about sustainability, the IPAC Leads who doubted the sustainability cited the transition to a new electronic health record system as a potential barrier.
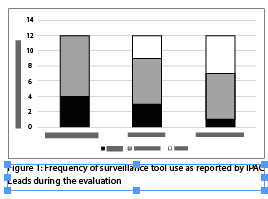
Although a need for improvement was identified, not all IPAC Leads used the daily surveillance form, case definitions or case validation forms consistently (Figure 1).
Frontline nurse evaluation
The electronic survey focusing on use of the daily surveillance form was completed by four nurses from 3/18 (16.7%) of the homes. All indicated that they had received adequate training and one respondent indicated that they only sometimes used the form consistently. All of the respondents reported spending less than 20 minutes per shift using this form, consistent with the perceptions of the IPAC Leads when asked about the time required by nurses to complete the daily surveillance form. All of the Leads had estimated that nurses spent less than 20 minutes per shift using the form.
The corporate Infection Control Professional and Health Informatics Coordinator (HICo) evaluation
The corporate Infection Control Professional (ICP) felt that the collaborative approach to developing the toolkit was a positive experience and that PHO support was necessary for the development and implementation of a robust surveillance program. They identified that transitioning the paper-based daily surveillance form to an electronic version could improve efficiency and expressed concern that the IPAC Leads were not always using the standardized case definitions consistently. The example provided was of a family insisting antibiotics be prescribed for a resident based on non-specific symptoms despite that resident not meeting any case definition for an infection. Pressure from the family prompted this resident to be considered a UTI case. Conversely, the corporate ICP also shared anecdotally that the rates of UTIs in the organization were trending downward, likely due to improved adherence to a standardized case definition.
The HICo suggested additional training may have improved the understanding of the need of using the tools consistently and that the case definitions were not being applied consistently because the Leads relied upon “common sense” to determine if a resident should be considered a case. It was felt that the Leads did not find the case validation forms useful and had suspected many were not using them. The HICo suggested that more comprehensive training may have improved the perception of usefulness of the tools.
The toolkit underwent a final revision based on the feedback from the final evaluation. The toolkit was reduced to four tools consisting of a guide, case definitions, the reporting tool and a training webinar. Responsibility of the toolkit was assumed by the IPAC Canada Surveillance and Epidemiology Interest Group in September 2020 and is available on the IPAC Canada website at: https://ipac-canada.org/surveillance-statistics-resources [12]. Additionally, an IPAC Canada hosted public webinar to share the toolkit was also presented on September 29, 2020 (available at https://ipac-canada.org/webinar-ltc-surveillance) [13].
Discussion
The aim of this study was to develop and trial a set of infection surveillance tools for use in LTC. Here, we described the successes and challenges encountered and identified ways to improve the toolkit for improved usability. The collaborative approach between a government agency with IPAC expertise and a LTC corporation providing input from front-line users was viewed as a positive and successful experience by the organization. The initial iteration of the toolkit was intended to be a comprehensive collection of tools that would meet the needs of a LTC home but a simplified, refined set of tools was preferred based on feedback gathered through informal and formal evaluations.
The data collected during the evaluation from nurses, the IPAC Leads, the HICo and the corporate ICP were classified into four themes: training, communication, engagement, and surveillance tool improvements.
Despite a full-day in-person training session and a series of webinars intended to support the implementation of the toolkit, the theme of training emerged as an area for improvement. A minority of the Leads reported using all of the tools consistently and only a quarter reported using the case definitions consistently. This suggests that the cases may not be reliably counted and rates, therefore, may not be comparable across the organization. The HICo suggested that additional training focusing on the importance of using the case definitions consistently may improve their application and the corporate ICP felt additional in-person training would have improved overall compliance. However, opportunities were limited given the geographical distribution of the homes. Additional in-person training for new staff hadn’t been offered so developing a strategy for sustainability when there is turnover in key positions within the organization is crucial to ensure consistent training and continued operation of the surveillance program.
Engagement of front-line nurses and IPAC Leads also emerged as a key theme. Engagement of the IPAC Leads in the final evaluation was low and 33% (6/18) were not successfully engaged to participate at all. This highlights the need to integrate flexible strategies that recognize heavy workloads and competing priorities. Additionally, only four nurses completed the electronic survey about use of the daily surveillance form. Barriers to completion of the survey may have included a general lack of engagement with the initiative, time constraints, lack of access to a computer or unclear messaging about the importance of participating in the evaluation. Addressing these potential barriers and providing additional support in developing engagement strategies may have improved compliance with completion of the survey. Additionally, engaging higher levels of leadership within the organization may have helped to ensure that expectations of participation are communicated and promoted.
There were challenges with communication identified throughout the project. The IPAC Leads had indicated that there was an upcoming transition to a new electronic health record system. Although this system would not replace the need for standardized surveillance tools, there was a perception that this system would impact the usefulness of the tools and may have resulted in reduced uptake of the toolkit. Ensuring that there are no competing process changes and improved communication about how the tools will compliment organizational processes may have helped the Leads understand the utility of the toolkit and improve uptake. The use of semi-automated or automated, standardized surveillance resources have been shown to reduce workload and save time and this benefit could also be highlighted to improve staff uptake [14, 15].
The evaluation provided some important feedback used to make improvements to the usability of the toolkit. The IPAC Leads shared their preference for having automated components of the surveillance reporting form and they had also reported that they did not find the case validation tool useful or easy to use. Revisions to the reporting form were made based on their preferences and the case validation tools were removed from the toolkit.
The heavy workloads and competing priorities of the IPAC Leads posed challenges during the trial of the surveillance toolkit. Having a dedicated IPAC role in each home may have improved uptake of the tools, as recently recommended by IPAC Canada [16]. One limitation of this study was the lack of verification that the surveillance system resulted in a reduction in HAIs. Despite an anecdotal decrease in the UTI rates noted, comparing trends, before and after implementation of the toolkit, may shed light on how the toolkit has affected infection rates over time. Another limitation noted was the low engagement of IPAC Leads in the evaluation process. The Leads who declined to be interviewed may have done so because of negative experiences with the toolkit or due to a general lack of prioritization of IPAC initiatives. Their feedback may have been useful in further improving the toolkit. This study was conducted in a LTC corporation with the support of a HICo and a corporate ICP. It is unclear if similar challenges would be encountered during implementation of the toolkit in a LTCH with a different level of resources. Increasing the number of LTCHs participating in the study may have contributed to more robust data and have identified additional barriers.
The results of this study are challenging to compare to similar quality improvement (QI) initiatives in LTCHs because interventions focusing on the reduction of HAIs in this setting seem to be rare. Development and implementation of an automated surveillance system was found to reduce workload and improve data reliability in a recent study in a hospital setting [14]. Another hospital-based study found that a standardize surveillance system improved the timeliness of reporting infections to health units, including analysis and presentation of infection data [17]. Further investigations into QI initiatives in LTC settings will help identify optimal interventions to further support robust surveillance systems and reduce the risk of HAIs in residents.
This project was successful in trialing a toolkit in a LTC corporation and using feedback gathered by multiple methods to create a revised and improved toolkit for the broader LTC sector. This toolkit can be used to collect, analyze and use standardized infection surveillance data in LTCHs in order to improve IPAC practices and reduce HAIs. Future work can include determining if the use of the surveillance toolkit led to interventions that resulted in the reduction of HAIs in residents and an assessment of sustainability and long-term IPAC lead satisfaction with the toolkit. The COVID-19 pandemic has highlighted the need for robust surveillance systems in LTCHs and the need for tools to support adherence to IPAC best practices is growing.
REFERENCES
1. Ontario Agency for Health Protection and Promotion (Public Health Ontario), Provincial Infectious Diseases Advisory Committee. (2014). Best practices for surveillance of health care-associated infections in patient and resident populations. 3rd ed. Toronto, ON: Queen’s Printer for Ontario. https://www.publichealthontario.ca/-/media/documents/B/2014/bp-hai-surveillance.pdf.
2. Haenan, A., Verhoef, L., Beckers, A., Gijsbers, E., Alblas, J., Huis, A., Hulscher, M., de Greeff, S. (2019). Surveillance of infections in long-term care facilities (LTCFs): The impact of participation during multiple years on health care-associated infection incidence. Epidemiology and Infection, 147, e266, 1-8. DOI: https://doi.org/10.1017/S0950268819001328.
3. Fixing Long-Term Care Act, SO 2021, c. 39, Sched 1. https://www.ontario.ca/laws/statute/21f39.
4. Gambeta, K., Chambers, A. (2021). Knowledge to action: Needs assessment to enhance support for infection control professionals across healthcare settings. Canadian Journal of Infection Control, 36, 86-93.
5. Arons, M., Hatfield, K., Reddy, S., Kimball, A., James, A. et al. (2020). Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. New England Journal of Medicine, 382,2081-2090. DOI: 10.1056/NEJMoa2008457.
6. Smith, D., Duval, A., Pouwels, K., Guillemot, Fernandes, J. et al. (2020). Optimizing COVID-19 surveillance in long-term care facilities: a modelling study. BMC Medicine, 18, 386. DOI: https://doi.org/10.1186/s12916-020-01866-6.
7. Toth, D., Khader, K. (2021). Efficient SARS-CoV-2 surveillance strategies to prevent deadly outbreaks in vulnerable populations. BMC Medicine, 19, 25. DOI: https://doi.org/10.1186/s12916-020-01886-2.
8. Ontario Agency for Health Protection and Promotion (Public Health Ontario), Provincial Infectious Diseases Advisory Committee. (2013). Annex A: Screening, Testing and Surveillance for Antibiotic-Resistant Organisms (AROs). Toronto, ON: Queen’s Printer for Ontario. https://www.publichealthontario.ca/-/media/documents/ /2013/aros-screening-testing-surveillance.pdf.
9. Ontario Agency for Health Protection and Promotion (Public Health Ontario), Provincial Infectious Disease Advisory Committee. (2013). Annex C: Testing, Surveillance and Management of Clostridium difficile in All Health Care Settings. Toronto, ON: Queen’s Printer for Ontario.
https://www.publichealthontario.ca/-/media/documents/ /2013/cdiff-testing-surveillance-management.pdf.
10. Stone, N.D. (2012). Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infection Control and Hospital Epidemiology, 33,10, 965–977.
11. Government of Canada. (2018). Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2. https://ethics.gc.ca/eng/policy- politique_tcps2-eptc2_2018.html.
12. IPAC Canada. (2020).Long Term Care Surveillance Toolkit in Collaboration with Public Health Ontario. https://ipac-canada.org/surveillance-statistics-resources.
13. Metcalf D. (2020). Surveillance of Infections in Long Term Care: An IPAC Canada Toolkit by Public Health Ontario. https://ipac-canada.org/webinar-ltc-surveillance.
14. Avina, K., & Sinha, R. K. (2022). Development of an Automated Hospital Infection Control Surveillance Toolkit. Journal of Health Management, 24,187-202. DOI: 10.1177/09720634221088442.
15. Verberk, J.D., Aghdassi, S. J., Abbas, M., Nauclér, P., Maldonado, N. et al. (2022). Automated surveillance systems for healthcare-associated infecitons: results from a European survey and experiences from real-life utilization. Journal of Hospital Epidemiology, 122, 35-43. DOI: https://doi.org/10.1016/j.jhin.2021.12.021.
16. IPAC Canada. (2021). Infection Prevention and Control (IPAC) Program Components for Long-term Care Homes (LTCHs). https://ipac-canada.org/photos/custom/pdf/positionStat_LTCH_28July2021_English.pdf.
17. Doell, L. (2007). Improving infection control, prevention and surveillance: Using the Electronic Health Record (EHR) in a 370-bed acute care hospital. American Journal of Infection Control, 35(5), E199-E200. DOI:
https://doi.org/10.1016/j.ajic.2007.04.223.


