Reprinted with permission from University of Toronto Press (https://utpjournals.press).
Lucy Y Eum BSc (Pharm), MD1, Stefanie Materniak BA, MSc (Epidem.)2, Paula Duffley RN3, Sameh El-Bailey MbChb, DCP, FRCPath4, George R Golding PhD5, Duncan Webster MA, MD, FRCPC6
1Department of Medicine, Dalhousie University, Halifax, Nova Scotia, Canada
2Horizon Health Network, Saint John, New Brunswick, Canada
3Infection Prevention and Control, Horizon Health Network, Saint John, New Brunswick, Canada
4Microbiology, Department of Lab Medicine, Horizon Health Network, Saint John, New Brunswick, Canada
5National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Manitoba, Canada
6Internal Medicine/Medical Microbiology, Dalhousie University, Saint John, New Brunswick, Canada
Corresponding author:
Lucy Y Eum
Department of Medicine, Dalhousie University
Suite 442, Bethune Building
1276 South Park Street
Halifax, Nova Scotia
B3H 2Y9 Canada.
Tel: 902-473-2379 | Email: lucy.eum@dal.ca
Contributors:
Conceptualization, S Materniak, D Webster; Methodology, S Materniak, D Webster; Software,S Materniak; Validation, S Materniak; Formal Analysis, S Materniak; Investigation, P Duffley; Resources, S El-Bailey, GR Golding, D Webster; Data Curation, S Materniak, P Duffley; Writing – Original Draft, LY Eum, S Materniak, D Webster; Writing – Review & Editing, LY Eum, S Materniak, P Duffley, S El-Bailey, GR Golding, D Webster; Visualization, LY Eum, S Materniak; Supervision, D Webster; Project Administration, S Materniak; Funding Acquisition, LY Eum, D Webster.
ABSTRACT
Background: Several decolonization regimens have been studied to prevent recurrent methicillin-resistant Staphylococcus aureus (MRSA) infections. Clinical equipoise remains with regard to the role of MRSA decolonization. We compared initial MRSA clearance and subsequent MRSA recolonization rates over a 12-month period after standard decolonization (using topical chlorhexidine gluconate, and intranasal mupirocin) or systemic decolonization (using topical chlorhexidine gluconate, intranasal mupirocin, oral rifampin, and oral doxycycline).
Methods: MRSA-colonized patients were randomized to receive either standard or systemic decolonization. Follow-up with MRSA screening was obtained at approximately three, six, and 12 months after completion of therapy. Kaplan-Meier survival curves were calculated and assessed for significant differences using log-rank tests.
Results: Of 98 enrolled patients (25 standard decolonization, 73 systemic decolonization), 24 patients (seven standard decolonization, 17 systemic decolonization) did not complete the study. Univariate analysis showed a marginally significant difference in the probability of remaining MRSA-negative post-treatment (p = 0.043); patients who received standard decolonization had a 31.9% chance of remaining MRSA-negative compared with a 49.9% chance among those who received systemic decolonization. With multivariate analysis, there was no difference in the probability of remaining MRSA-negative between systemic and standard decolonization (p = 0.165). Initial MRSA clearance was more readily achieved with systemic decolonization (79.1%; 95% CI 32.4% to 71.6%) than with standard decolonization (52.0%; 95% CI 69.4% to 88.8%; p = 0.0102).
Conclusions: Initial MRSA clearance is more readily achieved with systemic decolonization than with standard decolonization. There is no significant difference in the probability of sustained MRSA clearance.
KEYWORDS
decolonization, methicillin-resistant Staphylococcus aureus, MRSA, randomized controlled trial
ABSTRACT
Historique : Plusieurs schémas de décolonisation ont été étudiés pour prévenir la récurrence d’infections à Staphylococcus aureus résistant à la méthicilline (SARM). La pondération clinique demeure à l’égard du rôle de la décolonisation du SARM. Les chercheurs ont comparé la clairance initiale du SARM et les taux de recolonisation subséquents par le SARM sur une période de 12 mois après une décolonisation standard (au moyen de gluconate de chlorhexidine topique et de mupirocine intranasale) ou de décolonisation systémique (au moyen de gluconate de chlorhexidine topique, de mupirocine intranasale, de rifampine par voie orale et de doxycycline par voie orale).
Méthodologie : Des patients colonisés par le SARM ont été choisis au hasard pour recevoir une décolonisation standard ou systémique. Les chercheurs ont obtenu les données de suivi par un dépistage du SARM environ trois, six et 12 mois après la fin du traitement. Ils ont calculé la courbe de survie de Kaplan-Meier et l’ont évaluée pour déterminer les différences importantes au moyen des tests logarithmiques par rang.
Résultats : Des 98 patients inscrits (25 par décolonisation standard, 73 par décolonisation systémique), 24 n’ont pas terminé l’étude (sept par décolonisation standard, 17 par décolonisation systémique). L’analyse univariée a révélé une différence légèrement significative quant à la probabilité de demeurer négatif au SARM après le traitement (p = 0,043). En effet, les patients qui avaient reçu une décolonisation standard avaient 31,9 % de chances de demeurer négatifs au SARM, par rapport à 49,9 % de chances chez ceux qui avaient reçu une décolonisation systémique. À l’analyse multivariée, il n’y avait pas de différence entre la probabilité de demeurer négatif au SARM après une décolonisation systémique ou standard (p = 0,65). La clairance initiale du SARM était obtenue plus rapidement par la décolonisation systémique (79,1 %; IC à 95 % 32,4 % à 71,6 %) que standard (52,0 %; IC à 95 % 69,4 % à 88,8 %; p = 0,0102).
Conclusions : La clairance initiale du SARM est plus facile à obtenir par une décolonisation systémique que standard. La clairance initiale du SARM était obtenue plus rapidement par la décolonisation systémique que standard. Il n’y a pas de différence significative dans la probabilité de clairance soutenue du SARM.
MOTS-CLÉS
décolonisation, étude randomisée et contrôlée, SARM, Staphylococcus aureus résistant à la méthicilline
INTRODUCTION
Staphylococcus aureus is a virulent pathogen and an important cause of nosocomial and community-acquired infections in Canada, including skin and soft tissue infections, pneumonia, osteomyelitis, and bacteremia [1]. In the general population, 10%–30% of people are found to be persistently colonized with S. aureus [2]. S. aureus most commonly colonizes the anterior nares, but it is also found in the pharynx, vagina, rectum, and the skin, particularly cutaneous wounds and catheter exit sites [3,4].
Colonization with either methicillin-sensitive S. aureus (MSSA) or methicillin-resistant S. aureus (MRSA) has been consistently linked with subsequent infection [3,5,6]. A study by Butler-Laporte et al found that MRSA colonization predicted subsequent MRSA infection more accurately than any other risk factor for infection, including comorbidities, age, and number of previous hospitalizations [5]. MRSA infections are also associated with higher morbidity and mortality as well as with increased health care costs relative to MSSA infections [7,8]. Kourtis et al found that MRSA mortality rates remained higher than MSSA mortality rates between 2012 and 2017 [9]. In Canada, the incidence rate of community-associated MRSA bloodstream infections was shown to have increased between 2014 and 2018 [10], likely adding to the burden of MSSA infections rather than replacing the MSSA strains [11].
To prevent recurrent MRSA infections and transmission, various MRSA decolonization strategies have been investigated [4,12–17]. Although short-term staphylococcal decolonization appears feasible in many cases with topical regimens alone [4,13,14,18], certain populations, such as those with multiple-site MRSA colonization or extra-nasal colonization, were found to be more difficult to decolonize and to maintain decolonized status for an extended period of time with topical regimens alone [4,16,17]. Recent studies have found that the systemic decolonization regimen is more effective than the topical regimen alone at achieving initial clearance, especially for multiple or extra-nasal site eradication, although more randomized trials are needed [14,16,17]. In one Swedish study, Lindgren et al found significantly lower rates of decolonization in pharyngeal MRSA carriers who received topical treatment only, compared with systemic treatment, after a 6-month follow-up period [17]. The sustained effect of decolonization appears unclear because of differences in MRSA prevalence and variable follow-up periods between studies as well as high rates of study participants lost to follow-up [15–17].
The potential risks of systemic decolonization regimens include toxicity from medications and development of resistance [19]. Adverse reactions possibly related to systemic antibiotics (including dyspepsia, diarrhea, nausea, and vomiting) have been reported in staphylococcal decolonization studies, although the reactions are generally mild and lead to discontinuation of medications among few patients [15]. Previous studies on the development of antimicrobial resistance to select decolonization agents have yielded mixed results [13,19], and Tidwell et al found that antibiotic resistance is an ongoing concern with implementation of routine decolonization practices [14].
Given the potential risks and unclear long-term efficacy, clinical equipoise remains with regard to the role of systemic MRSA decolonization. A review by Simor found that identification of decolonization regimens with better prolonged efficacy data is needed [4]. Although Sharara et al found that staphylococcal decolonization in surgical and intensive care unit (ICU] patients reduced the risk of infection, studies with other patient populations have yielded mixed results [19]. Chase et al have highlighted the need for more randomized trials and longer-term follow-up data to ascertain the efficacy of systemic decolonization compared with topical decolonization [16].
The aim of this study was to compare the sustained MRSA decolonization rate over a 12-month period between patients on a standard regimen using chlorhexidine gluconate and intranasal mupirocin alone and those on a systemic regimen using topical chlorhexidine gluconate and intranasal mupirocin with oral rifampin and oral doxycycline. We were also interested in whether colonization sites at baseline could help better predict MRSA decolonization outcomes.
METHODS
Study population and setting
Patients in the study were assessed for enrolment and follow-up by an infection control registered nurse (RN) at the specialized Ambulatory MRSA Clinic at the Saint John Regional Hospital (SJRH) in Saint John, New Brunswick, between 2008 and 2018.
The SJRH is the largest tertiary care teaching hospital in New Brunswick, and the primary aim of the specialized clinic is the education and management of patients colonized or infected with MRSA in southern New Brunswick. This study was approved by the Horizon Health Network Research Ethics Board.
Potentially eligible patients were identified by the MRSA clinic RN, who was notified of positive MRSA screening results among both hospitalized and ambulatory patients. All cases were reviewed with the principal investigator (DW) at the time of enrolment. To be considered eligible for inclusion in the study, patients had to be aged 19 years or older and have a positive MRSA culture from at least one body site and no evidence of active infection at the time decolonization was initiated. MRSA isolates were confirmed to be sensitive to rifampin, tetracycline, mupirocin, and chlorhexidine before decolonization protocols were initiated.
Patients were excluded from the study if they were residents of a special care home or nursing home, awaiting placement in a long-term care setting, on any antimicrobial therapy for an active infection, allergic to any of the study medications, had known antimicrobial resistance to one of the study medications before the initiation of decolonization, unable to take medications by mouth or feeding tube, or pregnant or breastfeeding or if they had known hepatic cirrhosis or aberrant liver function.
Once informed consent was obtained, eligible patients underwent collection of baseline samples to screen for MRSA colonization in the anterior nares and rectum. Where applicable, samples from other previously positive body sites (i.e., skin lesions, throat, urine, and medical device exit sites) were also collected.
Study design
This was an open-label, randomized study comparing standard MRSA decolonization with systemic MRSA decolonization. Patients meeting all of the inclusion criteria and none of the exclusion criteria were randomized using a block randomization 1:3 allocation ratio. Blocks of 4, 8, or 12 were used in varying order. Randomized blocks were created using an online randomization program (https://www.sealedenvelope.com/simple-randomiser/v1/lists). An individual with no direct contact with or knowledge of the patient was contacted by the RN or physician managing the patient and provided with the patient’s treatment assignment. Patients assigned to the standard decolonization group received a seven-day course of daily 4% chlorhexidine gluconate body wash in combination with a 1-centimetre ribbon of 2% mupirocin ointment applied to the anterior nares twice daily with a cotton-tipped applicator. Patients assigned to the systemic decolonization group received a seven-day course of daily 4% chlorhexidine gluconate body wash and 2% mupirocin ointment applied to the anterior nares, in addition to 600 milligrams of oral rifampin daily and 100 milligrams of oral doxycycline twice daily. All participants were given instructions on treatment application by the Ambulatory MRSA Clinic RN via in-person meetings.
All participants were also given contact information for the RN should they have any questions. The RN also reviewed possible side effects. Treatment was started as soon as possible after a culture result indicated the presence of MRSA. To monitor compliance, patients were asked by the MRSA clinic RN to describe how they were administering the medications. They were also asked to report to the MRSA clinic RN if they noticed any of the side effects that were previously reviewed or any new changes or symptoms after starting the decolonization regimen, and these were documented by the RN.
Baseline demographic and other clinical information, including medical comorbidities, were gathered in patient interviews and through review of medical records. Baseline functional status was assessed using the Katz Index of Independence in Activities of Daily Living [20].
The primary outcome of this study was the rate of recolonization with MRSA over a 12-month follow-up period after completion of the standard regimen or the systemic regimen. Once participants were found to be recolonized, follow-up and collection of cultures were discontinued, such that recolonized participants were not followed up for the full 12 months. Recolonization was defined as any subsequent MRSA screen or clinical specimen from which MRSA was isolated after completion of assigned treatment. To assess the primary outcome, follow-up cultures for MRSA screening were obtained at approximately three months, 6 months, and 12 months after completion of therapy from the anterior nares and rectal region in addition to skin lesions, Foley catheter urine specimens, medical device exit sites, and any other prior MRSA-positive sites, when applicable. In addition, examination of whether colonization sites at baseline could help better predict outcomes was determined on a priori basis because previous studies found that certain populations, such as those with multiple-site MRSA colonization or rectal colonization, may be more difficult to decolonize and to maintain decolonized status for an extended period of time with topical decolonization alone [4,12,16,18].
The secondary outcome of this study was the initial clearance rate after completion of the standard regimen or the systemic regimen. Initial clearance rates were measured by obtaining three complete sets of MRSA cultures, each taken on separate days (with a median of seven days between each set and a range of 4–26 days) after the last day of either the standard or the systemic decolonization regimen, with no antibiotics for 48 hours before any of the sets. Cultures were taken from previously MRSA-positive sites as identified on baseline screening. Only patients who had negative results from all sites on all three sets were declared to have achieved initial clearance and were included in the assessment for the primary outcome.
Laboratory methods
S. aureus isolates were identified by routine laboratory procedures. MRSA was determined as per the Clinical and Laboratory Standards Institute (CLSI) guidelines [21], using an oxacillin screen plate assay. Any isolates exhibiting growth on the screen plate were further characterized by detection of the penicillin-binding protein 2' (PBP2') using the PBP2' latex agglutination test (Denka Seiken Co, Ltd, Tokyo, Japan) according to the manufacturer’s instructions. All S. aureus isolates that exhibited growth on the oxacillin screen plate and that were PBP2'-positive were considered to be MRSA. MRSA isolates obtained via submitted clinical specimens underwent susceptibility assays using VITEK instrumentation (bioM.rieux, Inc, Durham, NC, USA). VITEK cards (GP-67) for susceptibility assays were inoculated and incubated according to the manufacturer’s recommendations (bioM.rieux, Inc). MRSA isolates obtained through targeted screening were confirmed to be sensitive to rifampin, tetracycline, and mupirocin as per CLSI guidelines [22]. Susceptibility testing was not done on the positive follow-up cultures. A triplex real-time polymerase chain reaction was used as previously described for the detection of Panton-Valentine leucocidin (PVL), mecA, and species-specific nuc genes [22]. Isolates were molecularly characterized by sequence-based typing of the polymorphic region of the staphylococcal protein A (spa) gene [24] and assigned to a Canadian epidemic strain type, as previously described [22].
Statistical analysis
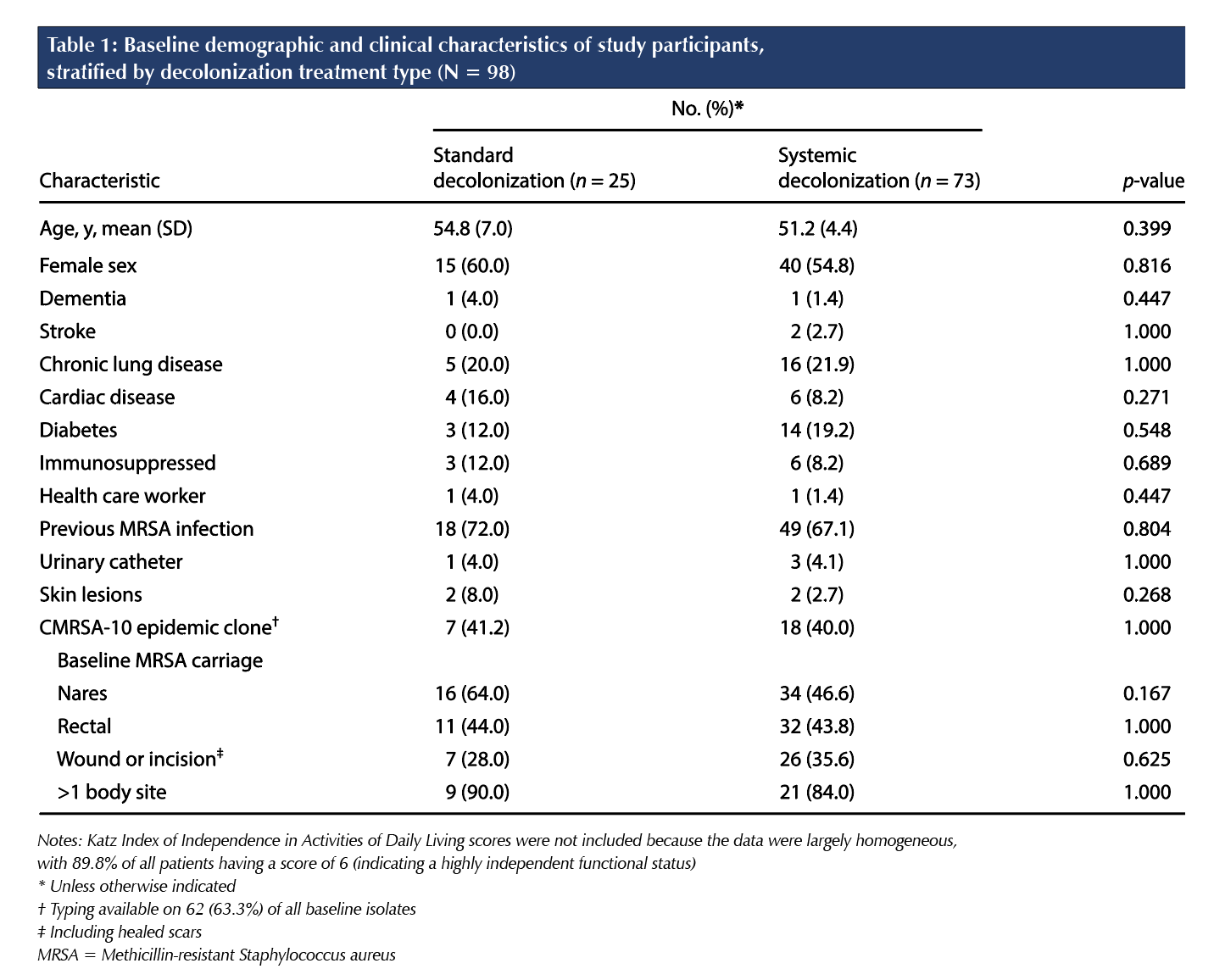
Data were stratified by treatment allocation to describe participant baseline characteristics in each group. Two-sample t tests and Fisher exact tests were used to describe whether significant differences (p < 0.05) were observed between the two groups.
To achieve 80% power for a two-group comparison of survivor functions log-rank test, Schoenfeld method with an ∂ of 0.05, a sample size of 100 participants in a 3:1 allocation ratio was used.
To examine the primary outcome of MRSA recolonization, follow-up time was defined as the time from the last day of decolonization treatment to the date on which the participant was found to be recolonized with MRSA. For individuals who did not have evidence of recolonization, follow-up time was from the end of treatment to the date of the last follow-up visit, due to death, loss to follow-up, or end of study (12 months post-treatment completion). Participants who began treatment but either did not complete treatment or had no subsequent follow-up had a follow-up time of 0. Intention-to-treat (ITT) analysis was used for the primary objective to obtain a more conservative estimate of treatment efficacy. Any patient who was randomized and began treatment is included.
MRSA recolonization was analyzed using Kaplan-Meier and log-rank tests to examine the crude likelihood of remaining MRSA-negative between standard and systemic decolonization. A Cox proportional hazards model was used to estimate the effect of the type of decolonization on the probability of remaining MRSA-negative using hazard ratios (HRs). The assumption of proportional hazards was tested using Schoenfeld residuals. Single-site rectal or nasal colonization or the presence of multi-site colonization at baseline was included in the final multivariate model on an a priori basis to examine whether colonization sites at baseline could help better predict outcomes. For the remaining variables, forward selection was used to examine for the effect of potential confounders identified during univariate analysis. Likelihood ratio tests (LRTs) were used to detect whether each potential confounder had a significant effect on the relationship between maintaining MRSA-negative status and decolonization treatment type. A p-value on the LRT of ≤ 0.05 was considered significant, and those variables were included in the final model. Results were reported using HRs and corresponding 95% confidence intervals (CIs). A two-tailed p < 0.05 was considered significant.
Analysis of the secondary outcome of achieving initial MRSA clearance between treatment arms was performed with a two-sample test of proportions or risk ratios (RRs) using Fisher exact tests. Stratified RRs used standard Mantel–Haenszel weights. All analysis was performed using Stata/IC (version 15.1; StataCorp, College Station, TX, USA).
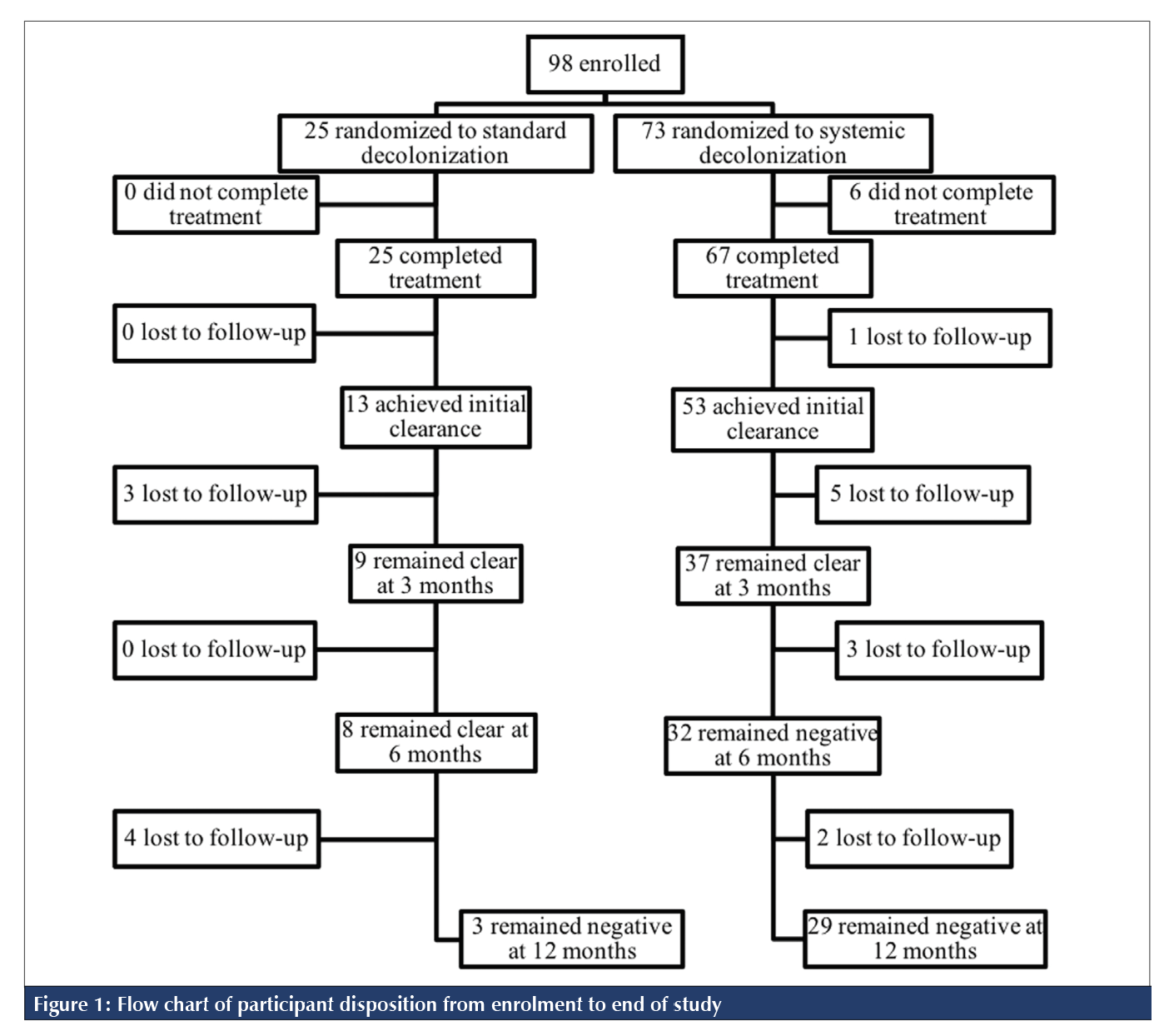
RESULTS
In total, 98 patients consented and were enrolled in the study; 25 patients (25.5%) were assigned to standard decolonization, and 73 patients (74.5%) were assigned to systemic decolonization (Figure 1). As seen in Table 1, baseline demographic and health-related characteristics did not significantly differ between the two treatment arms. Treatment was completed by 93.9% (n = 92) of participants. Treatment completion was 100.0% (n = 25) in the standard treatment arm and 91.8% (n = 67) in the systemic treatment arm. Of the 92 participants who completed treatment, 20.7% (n = 19; 7 in the standard group and 12 in the systemic group) were lost to follow-up. The proportion lost to follow-up in the standard and systemic treatment arms was not statistically significant (p = 0.2876).
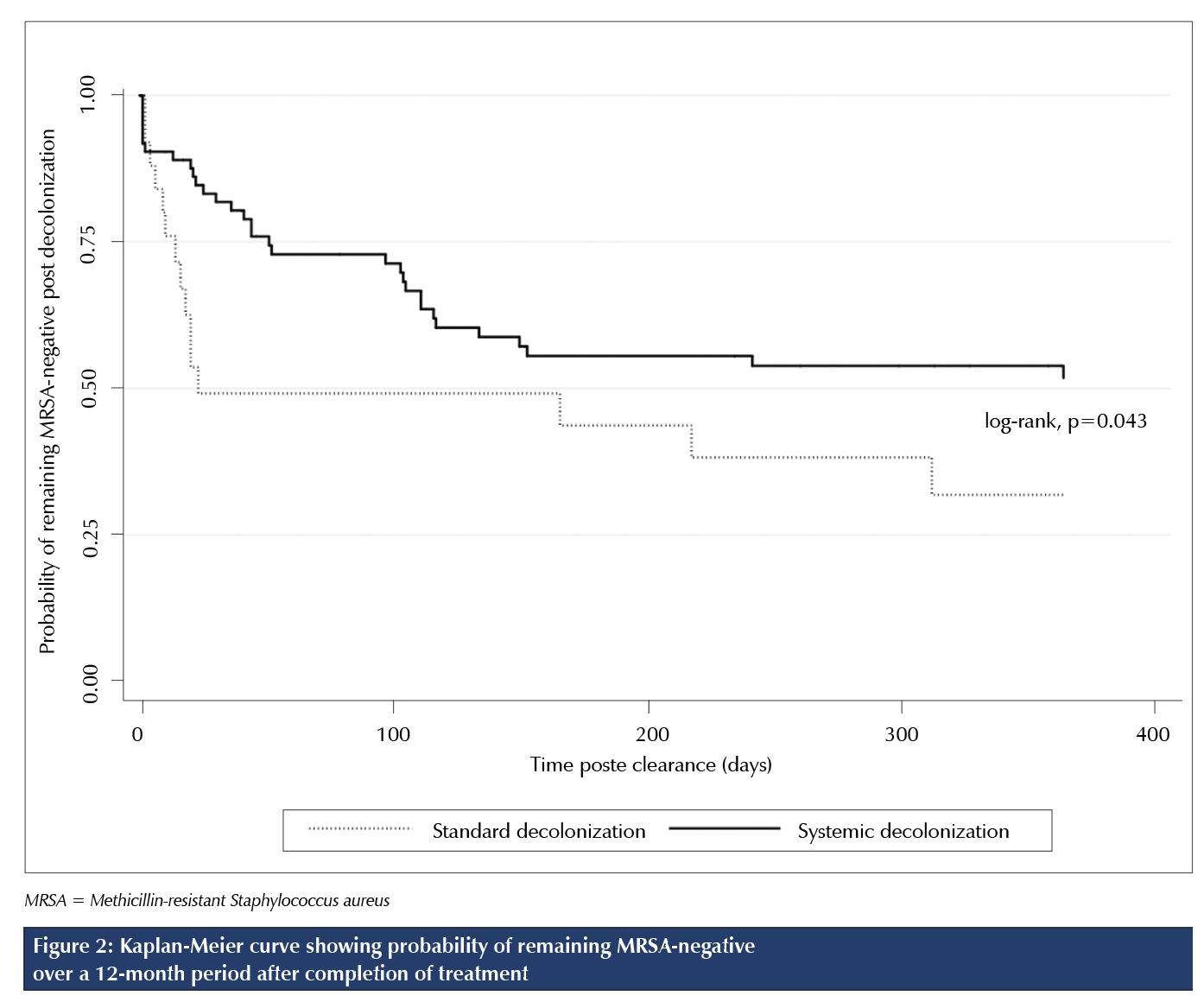
Of the 73 participants who were treated and completed the protocol to either reconversion or the 12-month follow-up, 56.2% (n = 41) were found either to continue to have MRSA present without achieving initial clearance (61.0%; n = 25) or to be recolonized with MRSA at follow-up (39.0%; n = 16). Median time to identification of recolonization post-treatment completion was 16 days (95% CI 6.5% to 22.5%) for standard decolonization versus 44 days (95% CI 22.0% to 104.0%) for systemic decolonization. As seen in the Kaplan-Meier survival curve (Figure 2), there was a marginally significant difference in the probability of remaining MRSA-negative post-treatment (p = 0.043). After 12 months of follow-up, univariate analysis showed a marginally significant difference in the probability of remaining MRSA-negative post-treatment (p = 0.043), where patients who received standard decolonization had a 31.9% chance of remaining MRSA-negative and patients who received systemic decolonization had a 49.9% chance. Of the 13 participants who achieved initial clearance on standard decolonization, 23.1% (n = 3) were found to be recolonized with MRSA at the 12-month follow-up. Of the 53 participants who achieved initial clearance on systemic decolonization, 26.4% (n = 14) were found to be recolonized with MRSA at the 12-month follow-up.
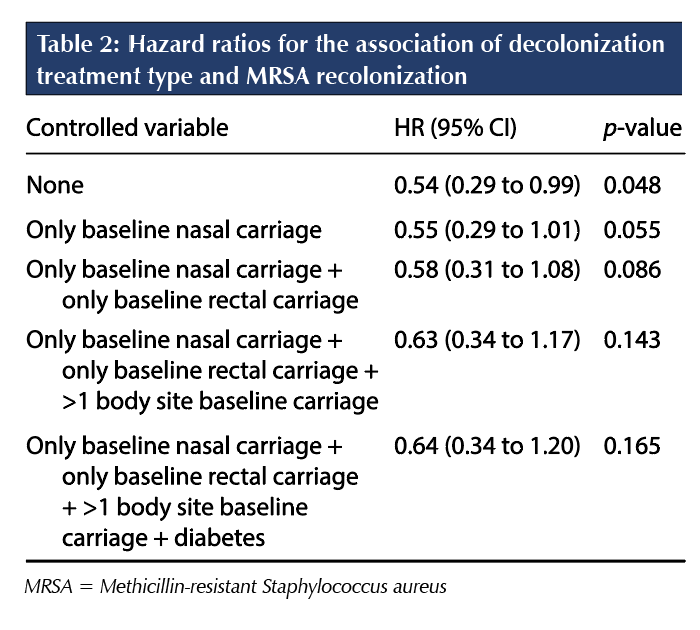
The crude effect of systemic decolonization on maintaining MRSA-negative status was only marginally significant (HR 0.54; 95% CI 0.29% to 0.99%; p = 0.048). Diabetes was identified as the only significant confounding variable and was included in the final model along with the a priori variables of single-site colonization in the nares or rectum and the presence of multi-site colonization. As shown in Table 2, once controlling for the effect of diabetes, single-site nasal or rectal colonization, and multi-site colonization, there was no longer a significant association between treatment assignment and maintaining MRSA-negative status (HR 0.64; 95% CI 0.34% to 1.20%; p = 0.165). Of the 32 patients who were recolonized with MRSA and had a baseline isolate typed, 12 (37.5%) had their recolonization isolate available for typing. Among these patients, all recolonization isolates were identical to their baseline type.
As our secondary outcome, we also found that the initial MRSA clearance rate was significantly higher with systemic decolonization (79.1%; 95% CI 32.4% to 71.6%) than with standard decolonization (52.0%; 95% CI 69.4% to 88.8%;
p = 0.0102). Using ITT analysis, there was no significant difference in the likelihood of achieving initial MRSA clearance (RR 1.40; 95% CI 0.93% to 2.09%; p = 0.0830). Taking into consideration the lack of typing on all participants at baseline, MRSA epidemic type (CMRSA-2 or CMRSA-10) did not affect the likelihood of initial clearance in ITT analysis (RR 1.16; 95% CI 0.74% to 1.81%; p = 0.3669).
MRSA isolates were only available for typing in 63.3% (n = 62) of patients; however, no significant difference was seen among the available samples in the proportions of CMRSA-2 (associated with USA100-800) and CMRSA-10 (associated with USA300) found in each treatment arm. Note that one patient was colonized with both CMRSA-2 and CMRSA-10 strains before undergoing decolonization. Overall, 58.7% (37 of 63) available isolates were CMRSA-2, and 41.3% (26 of 63) were CMRSA-10. All CMRSA-2 isolates were PVLnegative. One CMRSA-10 isolate was PVL-negative, and the remainder were PVL-positive.
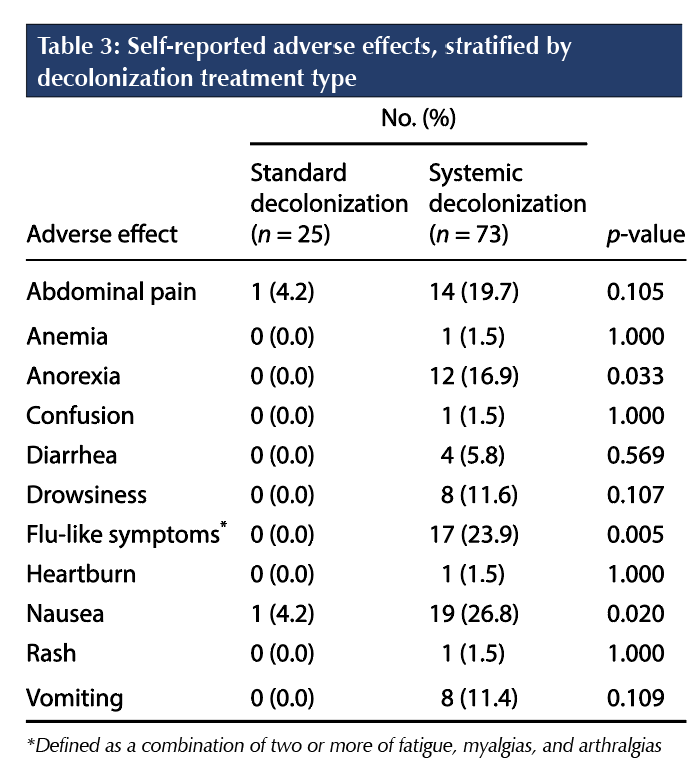
The six participants who discontinued treatment in the systemic arm were all due to reported side effects, with the most common being nausea (66.7%), flu-like symptoms (66.7%), and vomiting (60.0%). As seen in Table 3, those randomized to standard decolonization experienced far fewer adverse effects during treatment.
DISCUSSION
The results of the current study indicate that, after 12 months of follow-up, the rate of recolonization with MRSA was significantly higher with standard decolonization (4.9 patients per 1,000 follow-up days) than with systemic decolonization (2.3 patients per 1,000 follow-up days; 95% CI 0.25% to 0.85%; p = 0.011) before controlling for potential confounding variables, whereas there was no significant difference in the probability of sustained MRSA clearance between standard decolonization and systemic decolonization after controlling for the effect of diabetes, single-site nasal or rectal colonization, and multi-site colonization. Although univariate analysis showed a statistically significant difference in the probability of remaining MRSA-negative post-treatment (p = 0.043), where patients who received standard decolonization had a 31.9% chance of remaining MRSA-negative compared with patients in the systemic decolonization group, who had a 49.9% chance, there was no difference in the probability of remaining MRSA-negative between systemic and standard decolonization with multivariate analysis (p = 0.165). The initial MRSA clearance rate was significantly higher with systemic decolonization (79.1%; 95% CI 32.4% to 71.6%) than with standard decolonization (52.0%; 95% CI 69.4% to 88.8%; p = 0.0102).
Several MRSA decolonization strategies, using either topical antibiotics alone or a combination of both topical and systemic antibiotics, have been investigated [4,13–17,19]. Recent studies have found that topical and systemic combined decolonization regimens may be more effective than topical regimens alone at achieving initial clearance for certain populations [14,16,17,19].
Only two randomized controlled trials (RCTs) thus far have demonstrated efficacy of systemic MRSA decolonization regimens [15,17]. In a Canadian open-label, randomized trial by Simor et al, a seven-day regimen using the same decolonization protocols as this current study achieved a 92% initial clearance rate from all body sites, with 74% of patients remaining decolonized of MRSA for three months [15]. The results of this study were limited by the relatively short duration of follow-up and the lack of comparative efficacy data between systemic decolonization and standard (topical-only) decolonization [15]. A more recent Swedish RCT found that the proportion of participants who remained negative for MRSA was 40% higher in the group that received combined systemic and topical treatment than in the group that received topical-only treatment after six months of follow-up [17]. Limitations to that study included a relatively small sample size
(N = 69) and geographic differences in MRSA prevalence [17].
We also found that the initial MRSA clearance rate was significantly higher with systemic decolonization (79.1%; 95% CI 32.4% to 71.6%) than with standard decolonization (52.0%; 95% CI 69.4% to 88.8%; p = 0.0102). The high rate of initial clearance in the systemic decolonization group suggests that systemic decolonization may be of benefit in certain clinical settings that require immediate MRSA clearance, such as in
pre-operative settings or in the ICU [12,19].
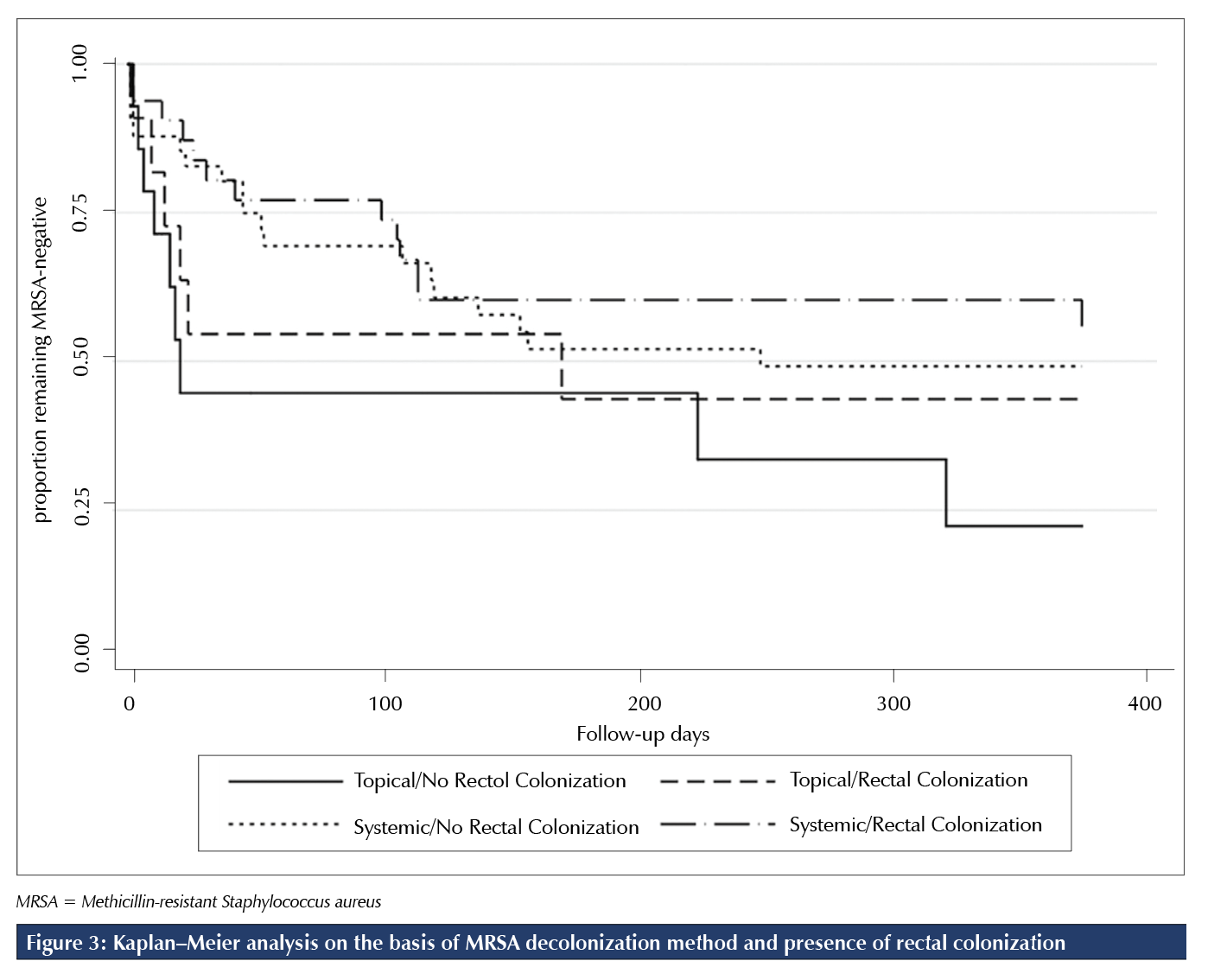
Previous studies also found that certain populations, such as those with multiple-site MRSA colonization or rectal colonization, may be more difficult to decolonize and to maintain decolonized status for an extended period with topical regimens alone [4,16,17]. A retrospective study conducted at our centre by Chase et al found that, among rectally colonized patients, the addition of systemic antibiotics to topical therapies resulted in higher rates of initial MRSA clearance and that prolonged maintenance of MRSA clearance was more readily achieved among those with non-rectal MRSA colonization than among those with rectal colonization [16]. To more closely examine the effect of rectal colonization on sustained MRSA clearance in the current study, we conducted a post hoc Kaplan-Meier analysis of the probability of remaining MRSA-negative on the basis of rectal colonization status and method of decolonization (Figure 3). This analysis found that the proportion of rectally colonized patients remaining MRSA-negative at 365 days was greater with systemic decolonization than with standard decolonization. However, it is important to note that this post hoc analysis was significantly underpowered. Strengths of the current study include the study’s RCT design, comparison of a systemic decolonization regimen with a standard regimen in efficacy and tolerability, and a longer follow-up period of 12 months compared with other randomized trials to assess sustained effect of decolonization. We also included patients in both ambulatory and acute care settings.
Limitations of this study should also be noted. This study was not a double-blind study, but this is unlikely to have affected the study outcomes because the primary and secondary outcomes were measured by obtaining cultures of pre-determined sites at pre-specified time intervals. Although patients were educated by our centre’s Ambulatory MRSA Clinic RN on the correct application of the topical interventions on enrolment, application techniques for the topical medications were not continuously monitored during the decolonization period, and this may limit the study’s validity. For many of the patients who were recolonized with MRSA during the follow-up period, we did not compare the MRSA genotypes of specimens obtained at baseline with those obtained at recolonization. It is unclear whether these individuals had a reduction in MRSA colonization burden with re-proliferation in subsequent weeks and months or whether they became newly colonized after clearance. More complete knowledge of the genotypes, including information on antimicrobial resistance in recolonization specimens through susceptibility testing, may have provided further insight.
The time required to recruit an acceptable number of patients in this study was long. After the study had commenced, providing standard decolonization became a standard-of-care option at the study site. As such, in many cases, using standard decolonization was desirable by the treating clinician and patient, and therefore many patients assessed in the Ambulatory MRSA Clinic opted to move forward with standard decolonization rather than enter into the randomized study. This led to low numbers of study participants over time and limited our ability to include a control group with no intervention for comparative data, despite the findings from previous trials that current evidence for benefit is limited to surgical and ICU patients [19]. Of note, in a multi-centre RCT comparing the effect of post-discharge hygiene education versus education combined with topical decolonization on MRSA infection rate, Huang et al found that post-discharge topical decolonization led to lower risks of infections and readmissions than hygiene education alone [13].
As we anticipated, patients were lost to follow-up over the course of the study. Although they appeared to have similar characteristics to those who completed the study, some unknown differences may not have been measured. This study excluded patients in or awaiting placement in a long-term care setting, so the results may not be generalizable to these populations. Given that Sharara et al found that staphylococcal decolonization among patients with recurrent skin and soft tissue infections, neonatal patients and their families, nursing home residents, and non-critically ill hospitalized patients yielded mixed results, a more inclusive study would be helpful [19]. Our study was a single-centre trial with a relatively small sample size. Findings should be interpreted with caution, especially in the context of inter-regional differences in MRSA prevalence and inter-institutional differences.
CONCLUSION
The results of this study show that the sustained effect of MRSA clearance is similar between standard decolonization and systemic decolonization after 12 months of follow-up. Initial MRSA clearance was more readily achieved with systemic decolonization than with standard decolonization. Both decolonization regimens were generally well tolerated, although significantly more nausea, anorexia, and flu-like symptoms were seen with systemic decolonization. For future studies, more RCTs examining the comparative efficacy and risks of MRSA decolonization strategies, especially using systemic agents, in various cohorts will be helpful.
REFERENCES
1. Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System 2017 report. 2017. https:// www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistancesurveillance-system-201seven-report-executive-summary.html (Accessed September 15, 2018).
2. Skar A, Br.geon F, Rolain J, Blin O. Staphylococcus aureus nasal decolonization strategies: a review. Expert Rev Anti Infect Ther. 2019;17(5):327–40. https://doi.org/10.1080/14787210.2019.1604220.
3. Botelho-Nevers E, Gagnaire J, Verhoeven PO, et al. Decolonization of Staphylococcus aureus carriage. Med Mal Infect. 2017;47(5):305–10. https://doi.org/10.1016/j.medmal.2016.10.005.
4. Simor AE. Staphylococcal decolonization: an effective strategy for prevention of infection? Lancet Infect Dis. 2011;11(12):952–62. https://doi.org/10.1016/S1473-3099(11)70281-X.
5. Butler-Laporte G, Cheng MP, McDonald EG, Lee TC. Screening swabs surpass traditional risk factors as predictors of MRSA bacteremia. BMC Infect Dis. 2018; 18(1):270. https://doi.org/10.1186/s12879-018-3182-x.
6. Abad CL, Pulia MS, Safdar N. Does the nose know? An update on MRSA decolonization strategies. Curr Infect Dis Rep. 2013;15(6):455–64. https://doi.org/10.1007/s11908-013-0364-y.
7. Thampi N, Showler A, Burry L, et al. Multicenter study of health care cost of patients admitted to hospital with Staphylococcus aureus bacteremia: impact of length of stay and intensity of care. Am J Infect Control. 2016;43(7):739–44. https://doi.org/10.1016/j.ajic.2015.01.031
8. Monaco M, Pimentel de Araujo F, Cruciani M, Coccia EM, Pantosti A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. Curr Top Microbiol Immunol. 2017;409:21–56. https://doi.org/10.1007/82_2016_3. PMID: 27025380.
9. Kourtis AP, Hatfield K, Baggs J et al. Vital signs: epidemiology and recent trends in methicillinresistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep. 2019;68(9):214–19. https://doi.org/10.15585/mmwr.mm6809e1.
10. Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System—update 2020. 2020. https://www.canada.ca/en/publichealth/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillancesystem-2020-report.html (Accessed May 1, 2021).
11. Mostofsky E, Lipsitch M, Regev-Yochay G. Is methicillin-resistant Staphylococcus aureus replacing methicillin-susceptible S. aureus? J Antimicrob Chemother. 2011; 66(10):2199–214. https://doi.org/10.1093/jac/dkr278.
12. Saraswat MK, Magruder JT, Crawford TC, et al. Preoperative Staphylococcus Aureus screening and targeted decolonization in cardiac surgery. Ann Thorac Surg. 2017;104(4):1349–56. https://doi.org/10.1016/j.athoracsur.2017.03.018.
13. Huang SS, Singh R, McKinnell JA, et al. Decolonization to reduce postdischarge infection risk among MRSA carriers. N Engl J Med. 2019;380(7):638–50. https://doi.org/10.1056/NEJMoa1716771.
14. Tidwell J, Kirk L, Luttrell T, Pike CA. CA-MRSA decolonization strategies: do they reduce recurrence rate? J Wound Ostomy Continence Nurs. 2016;43(6):577–82. https://doi.org/10.1097/WON.0000000000000277
15. Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44(2):178–85. https://doi.org/10.1086/510392.
16. Chase B, Webster D, Materniak S. Retrospective analysis of topical and topical/systemic combined methods of methicillin-resistant Staphylococcus aureus decolonization in an ambulatory patient population. Can J Infect Control. 2015;30(1):25–30.
17. Lindgren A, Nilsson AC, .kesson P, Gustafsson E, Melander E. Eradication of methicillin-resistant Staphylococcus aureus (MRSA) throat carriage: a randomised trial comparing topical treatment with rifampicin-based systemic therapy. Int J Antimicrob Agents. 2018;51(4):642–45. https://doi.org/10.1016/j.ijantimicag.2017.08.021.
18. Hanitsch LG, Krüger R, Hoppe P, et al. Outpatient decolonization after recurrent skin infection with Panton-Valentine leukocidin (PVL)-producing S. aureus—the importance of treatment repetition. PLoS One. 2020;15(4):e0231772. https://doi.org/10.1371/journal.pone.0231772.
19. Sharara SL, Maragakis LL, Cosgrove SE. Decolonization of Staphylococcus aureus. Infect Dis Clin North Am. 2021;35(1):107–33. https://doi.org/10.1016/j.idc.2020.10.010.
20. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the Index of ADL. Gerontologist. 1970;10(1 Part 1):20–30. https://doi.org/10.1093/geront/10.1_Part_1.20
21. Clinical Laboratory and Standards Institute. Performance standards for antimicrobial susceptibility testing: Sixteenth informational supplement. CLSI document M100-s16. Annapolis Junction, MD: CLSI; 2006.
22. Golding GR, Campbell JL, Spreitzer DJ et al.; Canadian Nosocomial Infection Surveillance Program. A preliminary guideline for the assignment of methicillin-resistant Staphylococcus aureus to a Canadian pulsed-field gel electrophoresis epidemic type using spa typing. Can J Infect Dis Med Microbiol. 2008;19(4):273–81. https://doi.org/10.1155/2008/754249.
23. McDonald RR, Antonishyn NA, Hansen T et al. Development of a triplex real-time PCR assay for detection of Panton-Valentine leucocidin toxin genes in clinical isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43(12):6147–9. https://doi.org/10.1128/JCM.43.12.614seven-6149.2005.
24. Harmsen D, Claus H, Witte W et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41(12):5442–8. https://doi.org/10.1128/JCM.41.12.5442-5448.2003.



