Caroline Etland, PhD, RN1, Linda Lybert1, Darrel Hicks, BA2, Master REH, CHESP2, Glenda Schuh, BSN, RN, CIC3,
Joanna Mills, MSN, CNS, CIC4, Jeanette Harris, MS, MSM, MT (ASCP), CIC, FACIP5, Ellen Turner, MBA1,6
1Healthcare Surfaces Institute, Stevensville, MT, US
2Environmental Services Specialist, Darrel Hicks Consulting, St. Louis, MO, USA
3Schuh & Associates, Longview, WA, USA
4John Muir Health, Walnut Creek, CA, USA
5Evergreen Health, Kirkland, WA, USA
6Sustainability and Specialty Additives, Synthomer, Kingsport, TN, USA
Corresponding author:
Caroline S. Etland, PhD, RN
President, Healthcare Surfaces Institute
Associate Professor, University of San Diego
Hahn School of Nursing and Health Sciences
5998 Alcala Park,
San Diego, CA 92110
Tel: 619-606-6581 | Email: cetland@sandiego.edu
ABSTRACT
Approximately 1.7 million infections occur annually in U.S. hospitals, with one in 25 patients acquiring a healthcare-associated infection (HAI) while hospitalized. Ongoing improvements in infection prevention and control protocols and processes reduce the risk of HAIs, but these are inhibited by surface pathogens that persist after routine cleaning and disinfection. It is unknown whether various surfaces and products in the healthcare setting are damaged by disinfectants creating invisible microbial reservoirs and ultimately, increasing the risk of transmitting pathogens. There are a variety of guidelines and recommendations to ensure equipment and surfaces can be cleaned and disinfected for safe use in the clinical setting, but no uniform approach exists for testing and product claims. Additionally, the life cycle of built environment surfaces and assembled clinical equipment may be greatly shortened by surface degradation, impacting cost and organizational sustainability goals. This consensus paper was developed to highlight gaps in evidence and recommend future action to a variety of stakeholders.
KEYWORDS
Healthcare surfaces, pathogen transmission, surface damage, cleaning and disinfection
INTRODUCTION
A healthcare facility must be a place designed to prevent harm to patients and deliver quality care in a safe environment. However, despite the best efforts of the healthcare profession, healthcare-associated infections (HAIs) continue to be a life-altering and even lethal threat to patients. According to the Centers for Disease Control and Prevention (CDC), about 1.7 million infections occur annually, with 99,000 associated deaths with one in 25 patients acquiring at least one HAI while being treated in a healthcare facility [1]. While ongoing improvements in infection prevention and environmental services protocols reduce the risk of HAIs, studies have demonstrated that these strategies are inhibited by surface pathogens that persist after routine and terminal cleaning and disinfection [2]. Thus, patients remain at risk for acquiring an HAI while hospitalized. Hence, a major barrier to creating a safe environment for patients and workers is the continuing lack of evidence on healthcare surfaces’ crucial role in harboring and spreading pathogens.
While progress has been made in mitigating the spread of airborne pathogens and increasing disinfection practices, the cleanability of surfaces is a fundamental area of concern that is sorely neglected and critical for ensuring the efficacy of these practices. The COVID-19 pandemic prompted healthcare professionals to become increasingly diligent about cleaning and disinfecting surfaces multiple times a day, but a critical question to be answered is whether these surfaces, materials, or products can be cleaned and disinfected using hospital-grade, Environmental Protection Agency (EPA)-registered disinfectants without causing damage. Once surface damage occurs, the true cost of product replacement becomes significant for healthcare facilities. It is also unknown how soon disinfectant-caused damage creates invisible microbial reservoirs in real-world settings, increasing the risk of transmitting pathogens [3].
There are many different surface materials and textiles in healthcare settings. It is rare that the built environment or any one product is made with only one surface material, so it is important to understand whether manufacturer guidelines indicate if all assembled materials can be cleaned and disinfected with the same product or method [4]. Otherwise, the disinfectant may cause microscopic damage to the surface and inhibit its cleanability [3, 5].
The surfaces selected and used on products and within the built environment are potential fomites for transmitting pathogens, yet little is known about the pathways and speed at which surfaces become contaminated and how quickly cross-contamination occurs. Huslage and colleagues introduced and defined the idea of high-touch surfaces without observing human behavior and understanding how people interact with surfaces [6]. The idea of prioritizing attention on high-touch surfaces for cleaning and disinfection proved to be somewhat effective, but there was conflict around what constitutes a high-touch surface, leading to inconsistencies in practice.
Therefore, a greater focus is needed on the design, construction, and operation of healthcare facilities and medical equipment to further reduce the spread of HAIs. In addition to creating facilities that are safer and more streamlined for better cleaning and disinfection, enhanced testing of product components pre-production is essential to mitigating surface degradation in real-world practice settings. Unfortunately, this type of testing lags behind the manufacture of new products and technology.
Many factors support microbial survival [7]. For example, environmental surfaces can support previously aerosolized pathogens and provide a safe harbor once attachment to surfaces occurs [8]. Humidity plays a key role, and there is evidence that certain types of microbes may grow on surfaces as biofilms [9-11]. A related concern is the availability of broad evidence that establishes firm links between construction and care of the built environment and reductions in HAIs [12, 13]. Indeed, the primary focus against infections has shifted from prevention to mitigation strategies using antimicrobial medications in the past few decades. However, widespread microbial resistance to antibiotics and other chemotherapies are forcing a revival of interest in preventive strategies including environmental cleaning and disinfection, implementation of cleanable surfaces, and ensuring product compatibility.
Damage to surfaces may occur at a microscopic level from cleaning and disinfection processes, thus compromising routine decontamination practices and increasing potential health risks to patients [3, 14]. A greater research emphasis is needed regarding compatibilities between surface materials and disinfectants, as well as research on the potential of surfaces to support microbial growth. In addition, common terms such as “clean” and “disinfectant” remain ill-defined, meaning different things to different stakeholders such as manufacturers, infection preventionists, and regulators/policymakers.
Increasing the complexity of the problem is the wide variety of non-porous and porous materials (e.g., countertops and textiles) within healthcare settings. There are also devices and medical/surgical equipment that require their own cleaning and disinfection protocols. Many such devices are a composite of materials requiring their own Instructions for Use (IFUs) for cleaning, disinfecting or reprocessing.
In summary, issues surrounding environmental surfaces and healthcare devices lack the necessary research and evidence to produce clear, unambiguous guidance, and need to be collaboratively addressed by a variety of disciplines in a meaningful and scientifically robust manner. Sustainable infection prevention solutions will not be found until healthcare surfaces become a primary focus of research and innovation.
Consensus Development Process
In 2015, the first Healthcare Surfaces Summit was held to discuss the many issues regarding surfaces, cleaning and disinfection, and HAIs. The Summit’s goal was to bring together a diverse group of healthcare professionals, academic and industry researchers, and manufacturing representatives to understand this issue and propose solutions. Some 30 experts representing infection preventionists, nursing professionals, academics, facility managers, and manufacturers were invited to participate. It was agreed that systematic efforts are needed to close the identified knowledge gaps, strategize research priorities, and develop consistent guidelines. In 2016, the Healthcare Surfaces Institute (HSI) was incorporated as a 501(c)(3) non-profit organization with the mission of addressing the evidence gaps and achieving consensus regarding priorities and solutions for industry, academia, and the service sectors. Analysis of published research has led to the creation of this consensus paper.
State of the Science
The wide variety of perspectives and backgrounds of the group members was valuable to understanding the state of the science for healthcare surfaces. It quickly became evident that a literature review was necessary to identify gaps in the evidence and plan a research agenda. A systematic review of the literature was undertaken in collaboration with several university faculty members who were also members of the HSI Board of Directors. The scope of the review was broad rather than a focused clinical question. Scientific literature databases were searched and evidence was gathered to answer the question, “What role do environmental surfaces play in the transmission of pathogens in healthcare settings?” Research reports published in peer-reviewed journals were retrieved and analyzed by the systematic review team. Of note, many publications exist from a wide variety of organizations and individuals that provide high value in understanding the role of surfaces in the transmission of pathogens. However, most of these publications exist in the “grey literature,” outside of the control of commercial publishers, and were not evaluated by blinded reviewers [15]. More recent systematic reviews reflect the rapid increase of publications regarding surfaces [16].
Interestingly, several international work groups have made progress in addressing related issues. However, none have convened a group to address surfaces broadly. Peters and colleagues reported using a similar approach to gaining consensus during the Healthcare Cleaning Forum at the Interclean trade show in Geneva, Switzerland [17]. Roques and colleagues had previously identified similar concerns and priorities in a consensus paper from a European workgroup [18]. The American Industrial Hygiene Association (AIHA) published a focused white paper on disinfection practices using germicidal ultraviolet radiation in the workplace [19]. Besides defining key terms, the white paper reiterated recommendations to prevent adverse effects from worker exposure to ultraviolet radiation with Threshold Limit Values (TLVs).
Peters and colleagues provided a glossary of terms related to hospital cleaning to facilitate understanding of their recommendations [17]. These definitions allowed for consistency during forum discussions. However, there is little consensus in the literature or published recommendations and guidelines on definitions of key terms. The consensus paper from Roques and colleagues in 2015 noted the same “lack of a common language or lexicon” to define terms and recommended clarification of terms be a primary priority of expert workgroups [18]. The lack of consensus on definitions of key terms (such as “clean” and “disinfected”) has exacerbated the confusion surrounding recommendations from different organizations. In fact, over 30 professional organizations have disseminated guidelines on cleaning and disinfection of surfaces in healthcare environments.
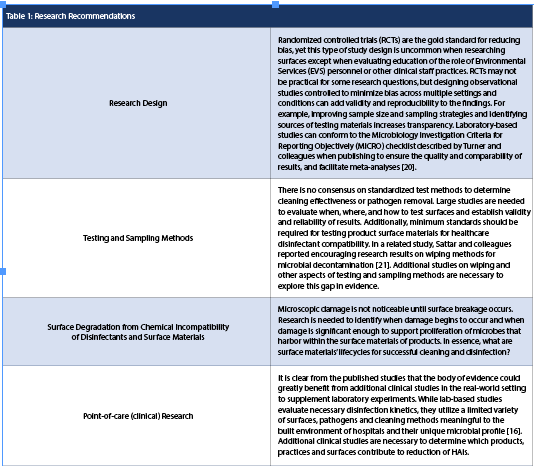
Recommendations
The consensus group agreed that several general recommendations would be helpful to constituents, as well as specific stakeholder calls to action. General recommendations will be discussed first, followed by research design recommendations (Table 1) and suggested actions for specific stakeholders (Table 2).
General Recommendations
Identify or revise new/existing guidelines for testing, cleaning and disinfection, and other terms. Standardized guidelines and terminology should be proposed, reviewed, and disseminated to stakeholders for comment and organizational adoption. This may serve to reduce disparities in understanding and practices between different areas of expertise. Another recommendation is to standardize the template for IFUs to improve readability, clarity and consistency for multiple healthcare roles involved in cleaning and disinfection.
Research Recommendations
HSI’s literature review identified key areas of evidence lacking in the literature, with priorities affirmed by expert consultants working in each field. It was observed that although literature concerning cleaning and disinfecting practices exists, there are significant gaps in knowledge regarding surface-disinfectant compatibility. Future research can help validate and provide support for product claims regarding surface-disinfectant compatibility in clinical settings. Equally important is the need to identify healthcare partners to participate in clinical site research that produces new knowledge from which leaders can make informed decisions.
SUMMARY AND CONCLUSIONS
Although some progress to advance knowledge on the role of healthcare surfaces in the transmission of pathogens has occurred, much work remains to be done. Collaborative efforts between researchers, manufacturers, healthcare professionals, regulatory/policy leaders, and advocates can build upon the excellent work to date. Cost-effective solutions to patient and employee safety issues can be supported by the enhancement of products used in the healthcare setting, bolstered by advances in science, manufacturing, and healthcare facility design. In addition, healthcare facilities executives can lead sustainable change by incorporating evidence into decision making.


REFERENCES
1. Centers for Disease Control and Prevention. (2017, December 14). Healthcare-associated Infections (HAIs). https://www.cdc.gov/winnablebattles/report/HAIs.html.
2. Weber, D., Anderson, D., & Rutala, W. (2013). The role of surface environment in healthcare-associated infection. Current Opinion in Infectious Disease, 26(43), 338-e44. https://doi.org/10.1097/QCO.0b013e3283630f04.
3. Jo, H., West, A., Teska, P., Oliver, H., & Howarter J. Assessment of early onset surface damage from an accelerated disinfection protocol. (2019). Antimicrobial Resistance and Infection Control, 8(24), 1-10. https://doi.org/10.1186/s13756-019-0467-9.
4. Centers for Disease Control and Prevention. (2008). Factors Affecting the Efficacy of Disinfection and Sterilization: Guideline for Disinfection and Sterilization in Healthcare Facilities. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html.
5. Teska, P., Dayton, R., Li, X., Lamb, J., Strader, P. (2020). Damage to common healthcare polymer surfaces from UV exposure. Nano LIFE, 10(3). https://doi.org/10.1142/S1793984420500014.
6. Huslage, K., Rutala, W., Sickbert-Bennett, E., & Weber, D. (2010). A quantitative approach to defining "high-touch" surfaces in hospitals. Infection Control & Hospital Epidemiology, 31(8), 850-853. https://doi.org/10.1086/655016.
7. Centers for Disease Control and Prevention. (2018). Factors Affecting the Efficacy of Disinfection and Sterilization: Guideline for Disinfection and Sterilization in Healthcare Facilities. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html.
8. Stone, W., Kroukamp, O., Korber, D., McKelvie, J., & Wolfaardt, G. (2016). Microbes at surface-air interfaces: The metabolic harnessing of relative humidity, surface hygroscopicity and oligotrophy for resilience. Frontiers in Microbiology, 1563, 1-15. https://doi.org10.3389/fmicb.2016.01563.
9. Bonadonna, L., Briancesco, R., Coccia, M. Analysis of microorganisms in hospital environments and potential risks. In: Capolongo, S., Settimo, G., Gola, M. (Eds.) Indoor Air Quality in Healthcare Facilities. SpringerBriefs in Public Health. https://doi.org/10.1007/978-3-319-49160-8_5. Published 2017.
10. Jablonska-Trypuc, A., Makula. M., Wlodarczyk-Makula. M., Wołejko, E., Wydro, U., Serra-Majem, L., & Wiater, J. (2022). Inanimate surfaces as a source of hospital infections caused by fungi, bacteria and viruses with particular emphasis on SARS-CoV-2. International Journal of Environmental Research and Public Health, 19(13), 8121. http://doi.org/10.3390/ijerph19138121.
11. Espinal, P., Marti, S., & Vila, J. (2012). Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. Journal of Hospital Infection, 80(1), 56-60. http://doi.org/https://doi.org/10.1016/j.jhin.2011.08.013.
12. Agency for Healthcare Research and Quality. (2013). Understanding the role of health care facility design in the acquisition and prevention of HAIs. (AHRQ Publication No. 13-0053-EF). U.S. Department of Health and Human Services. https://www.ahrq.gov/sites/default/files/publications/files/haidesign-summary.pdf
13. Jacob, J., Kasali, A., Steinberg, J., Zimring, C., & Denham, M. (2013). The role of the hospital environment in preventing healthcare-associated infections caused by pathogens transmitted through the air. (2013). Health Environments Research & Design Journal, 7(Suppl 1), 74-98. https://doi.org/10.1177/193758671300701S07.
14. Strader, P., Lee, Y., Teska, P., Li, X., & Jones, J. (2019). Approaches for characterizing surfaces damaged by disinfection in healthcare. Nano Life, 9(4). https://doi.org/10.1142/S1793984419500028.
15. Paez, A. (2017). Grey literature: an important resource in literature reviews. Journal of Evidence-Based Medicine, 10(3), 233-240. https://doi.org/10.1111/jebm.12266.
16. Christenson, E., Cronk, R., Atkinson, H., Bhatt, A., Berdiel, E., Cawley, M., Cho, G., Knox, C. K., Coleman, Harrington, C., Heilferty, K., Fejfar, D., Grant, E. G., Grigg, K., Joshi, T., Mohan, S., Pelak, G., Shu, Yl, & Bartram, J. (2021). Evidence map and systematic review of disinfection efficacy on environmental surfaces in healthcare facilities. International Journal of Environmental Research and Public Health, 18(21), 11100. https://doi.org/10.3390/ijerph182111100.
17. Peters, A., Otter, J., Moldovan, A., Parneix, P., Voss, A., & Pittet, D. (2018). Keeping hospitals clean and safe without breaking the bank: Summary of the Healthcare Cleaning Forum 2018. Antimicrobial Resistance & Infection Control, 7(132), 2-12. https://doi.org/10.1186/s13756-018-0420-3.
18. Roques, C., Al Mousa, H., Duse, A., Gallagher, R., Koburger, T., Lingaas, E., Petrosillo, N., & Škrlin, J. (2015). Consensus statement: patient safety, healthcare-associated infections and hospital environmental surfaces. Future Microbiology, 10(10), 1629-1634. https://doi.org/10.2217/fmb.15.85.
19. Fuller, T., Phillips, M., Davis, C., Fontaine, B., & Ratliff, J. (2021). Occupational safety and health guide for surface disinfection practices using germicidal ultraviolet radiation version 3 [White paper]. American Industrial Hygiene Association. https://aiha-assets.sfo2.digitaloceanspaces.com/AIHA/resources/Occupational-Safety-and-Health-Guide-for-Surface-Disinfection-Practices-using-Germicidal-Ultraviolet-Radiation-White-Paper.pdf.
20. Turner, P., Fox-Lewis, A., Shrestha, P., Dance, D. A. B., Wangrangsimakul, T., Cusack, T., Ling, C. L., Hopkins, J., Roberts, T., Limmathurotsakul, D., Cooper, B. S., Dunachie, S., Moore, C. E., Dolecek, C., Rogier van Doorn, H., Guerin, P. J., Day, N. P. J. & Ashley, E. A. (2019). Microbiology Investigation Criteria for Reporting Objectively (MICRO): a framework for the reporting and interpretation of clinical microbiology data. BMC Medicine, 17(1), 1-8. https://doi.org/10.1186/s12916-019-1301-1.
21. Sattar, S. A., Zargar, B., & Naderi, S. (2019). A carrier platform to test microbial decontamination of high-touch environmental surfaces by wiping. Healthcare Hygiene Magazine, 1(1), 32-33. https://viewer.joomag.com/healthcare-hygiene-magazine-october-2019/0530984001570139131?page=4.
22. Boyce, J., & Pittet, D. (2002). Guideline for hand hygiene in health-care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force.
Society for Healthcare Epidemiology of America/Association of Professionals in Infection Control/Infectious Diseases Society of America. MMWR Morbidity and Mortality Weekly Report, 51(RR-16), :1-44. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5116a1.htm



