Jörn Klein, PhD;a* Bjarne Hjeltnes, MSc;b Hege Tunsjø, PhD;b Colin Charnock, PhDb
a Faculty of Health and Social Sciences, University College of Southeast Norway
b Faculty of Health Sciences, Oslo and Akershus University College of Applied Sciences
Corresponding author
Joern Klein, PhD, University College of Southeast Norway, Faculty of Health and Social Sciences, Department of Nursing and Health Sciences, Postboks 235, 3603 Kongsberg, Norway
This email address is being protected from spambots. You need JavaScript enabled to view it.
ABSTRACT
Background: There is an increasing use of assistive technology and interactive therapeutic robots in nursing homes. However, little is known about the possible risks for transmitting infectious diseases through the use of such devices.
Methods: Representative surface samples of two multipurpose hygiene chairs and two interactive therapeutic robots were collected on a weekly basis at two nursing homes over a period of two months.
Results: We found that both robots and hygiene chairs may contribute to pathogen transmission.
Key words
Assistive technology, interactive therapeutic robots, HAI, multipurpose hygiene chairs, nursing home
BACKGROUND
Norwegian municipalities are increasingly using assistive technology and interactive therapeutic robots in their nursing homes [1]. Some of these products come in close physical and protracted contact with several patients and might constitute a source of infection. Little is known about the possible risks for transmitting infectious diseases through these devices. In this study we focused on multipurpose hygiene chairs and PARO interactive therapeutic robots.
Multipurpose hygiene chairs are used for washing and cleaning routines that require assistance from nursing staff (Figure 1).
PARO robots (Figure 2) are used in dementia care [2] to stimulate patients and cleaning done by the nursing home staff can only be done in a superficial way. Washing the interactive robot is not possible so that the artificial fur needs to be replaced by the distributor.
We collected representative surface samples of two hygiene chairs and two robots on a weekly basis over a period of two months at two nursing homes and analyzed the samples for the presence of clinically relevant microorganisms.
MATERIAL & METHODS
Nursing homes
Two nursing homes of approximately the same size, but located in different municipalities and with slightly different management structures took part in the study. Both nursing homes have implemented infection control programs.
Multipurpose hygiene chairs and PARO robots
Four hygiene chairs (Carendo, ArjoHuntleigh, Sweden), two in each nursing home, were labeled according to the following scheme NxCx (N for nursing home 1 or 2, C for chair 1 or 2). N1C1 was not in use, due to necessary maintenance, but served as reference. N2C2 had been used by one resident only. N1C2 and N2C1 were in use by more than one resident, and no special precautions other than visible cleaning have been done. All hygiene chairs were visible clean according to applied standards [3] before sampling.
Four PARO robots, two in each nursing home, labeled NxPx (N for nursing home 1 or 2, P for robot 1 or 2). N1P1 and N1P2 were in sporadic use during the sampling period. For all PARO robots, there was no cleaning performed between the use by different residents.
 Swab sampling
Swab sampling
Sterile flocked swabs were moistened in sterile water prior to surface swabbing of approximately 100 cm2. Two duplicate samples were taken each time and stored in either sterile water for bacterial cultivation or RNAlater for PCR analysis respectively.
Duplicate surface samples were taken with the M40 Transport system for bacterial cultivation.
ATP analysis
Duplicate ATP surface samples were taken according to the manufacturer’s protocol (Hygiena, UltraSnap™ surface test).
Contact sampling with dry nutrient medium plates
Duplicate surface contact samples were taken with Rida®Count test plates for total bacteria and Staphylococcus aureus counts.
MRSA
Staphylococcus aureus colonies from Rida®Count Staph. aureus test plates were transferred to MRSASelect™ agar (BioRad).
Bacteriology from swab samples
Duplicates swab samples were pooled and transferred to the following selective media:
- E. coli/coliform and ESBL detection: Brilliance E.coli/coliform selective Agar and ESBL agar (Oxoid).
- Enterococci and VRE: HiCromeTM Rapid Enterrococi agar, VancoScreening Brain Heart Infusion agar (NordicAST).
- MRSA: Samples were grown for 48 hours in PHMB enrichment broth without cefoxitin, [4] and screened for MRSA. Results have been validated by PCR [5].
- Clostridium difficile: Samples were grown anaerobically in CCFT-broth [6] and plated on Braziers Clostridium difficile selective agar, after two and ten days. Results have been validated by PCR [7].
Antibiotic resistant strains were identified by MALDI-TOF MS Biotyper (Bruker Daltonik, Germany).
PCR
qPCR was performed for influenza A and norovirus 1 and 2 were done as described in [8].
RESULTS
PARO robots N1P1 and N1P2 were not in daily use, which could explain the lower arithmetic mean relative light units (RLU) values, i.e., luciferase activity, compared to the frequently used N2P1 and N2P2 (Figure 3, a). By contrast, N1P1 and N1P2 gave higher arithmetic mean CFU for both aerobic (Fig. 3, b) and Staphylococcus aureus counts (Fig. 3, c).
One sampling of N2P2 gave a single atypical colony on the MRSASelect™ agar. This colony was subsequently identified as methicillin resistant Staphylococcus epidermis by MALDI-TOF.
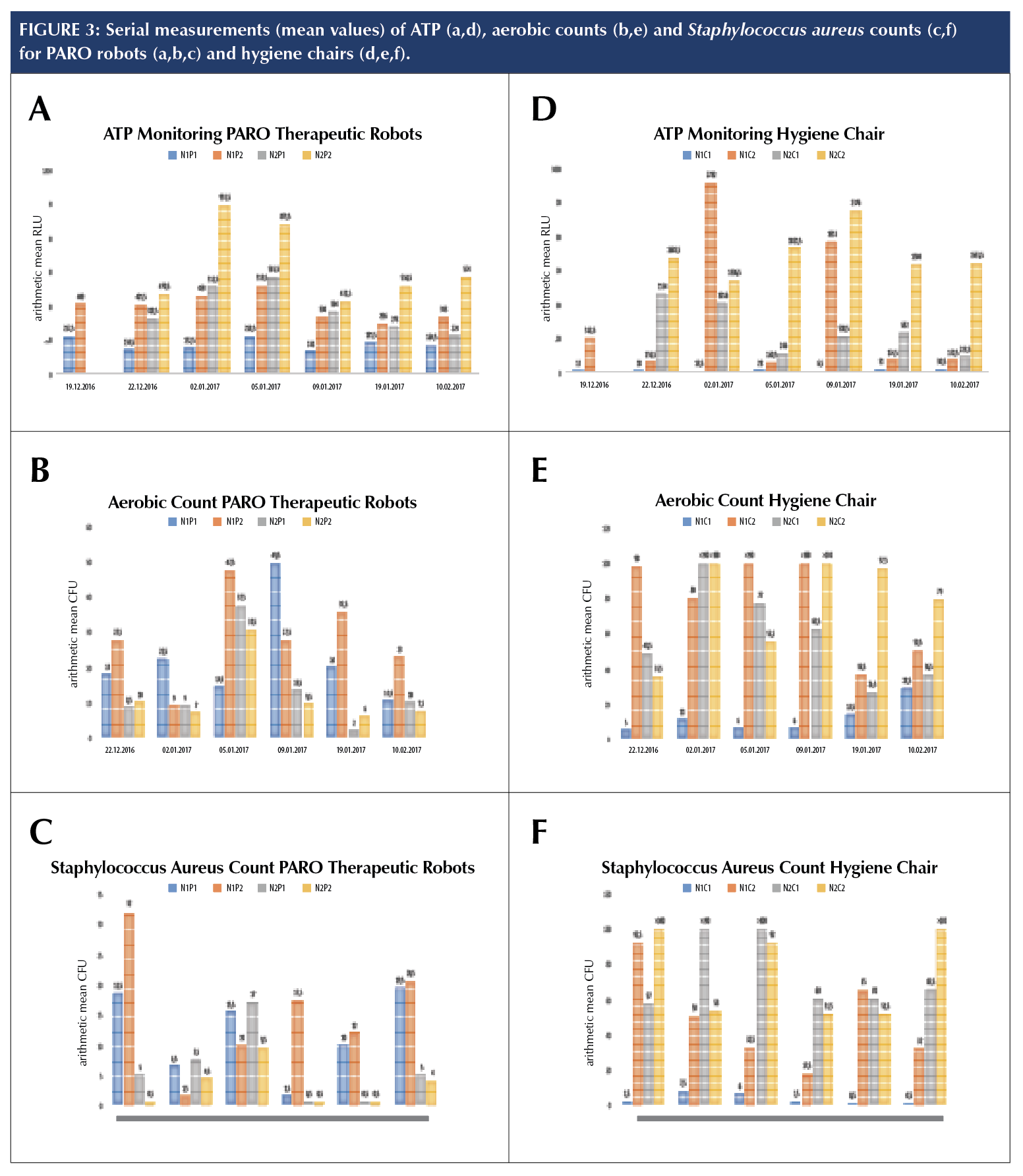
The hygiene chair N1C1 was not in use at the time of testing which may explain the results of the ATP monitoring. However the aerobic count had an arithmetic mean CFU/ml up to 28.5 (Fig. 3). All other Hygiene chairs were sampled after standard cleaning. These showed both a high aerobic count (Fig. 3, e), as well as a higher degree of contamination with Staphylococcus aureus (Fig. 3, f). Furthermore, the ATP monitoring (Fig. 3, d) revealed that biological contamination in nursing home two was higher overall than in nursing
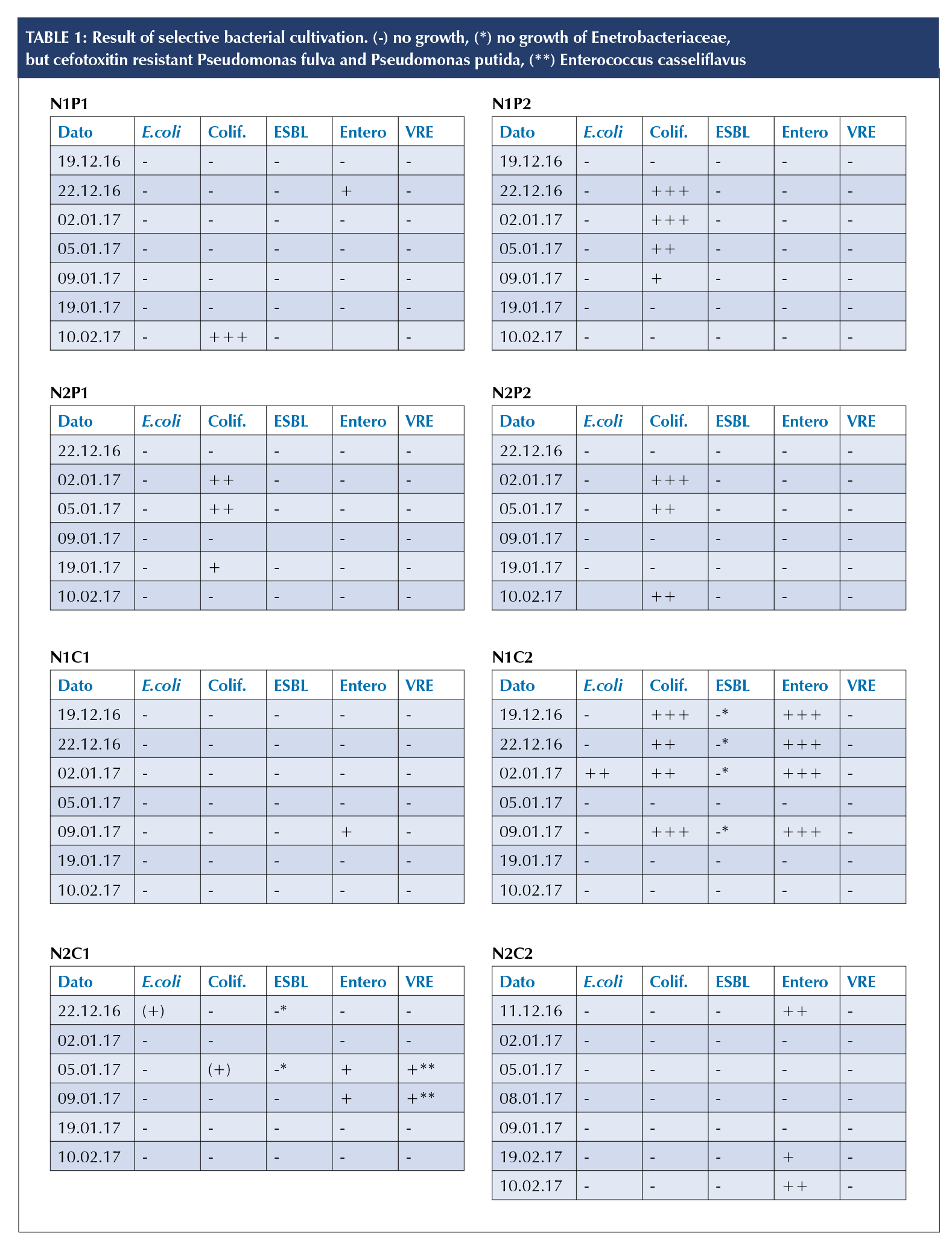
home one. One Rida®Count sampling of N2C1 gave typical colonies for MRSA on the MRSASelect™ agar. The latter was, however, not validated by other methods. Coliform bacteria (Table 1) were found on all four robots. N1P1 tested positive for Enterobacteriaceae at one sampling (Table 1, N1P1).
Except for N1C2, all hygiene chairs showed little or no E. coli or other coliform bacteria (Table 1). N2C2 tested positive for Enterobacteriaceae. Cefoxitin resistant Pseudomanas fulva and Pseudomanas putida were found on samples obtained from N2C1 and N1C2. N2C1 tested positive for a vancomycin-resistant enterococci (VRE), namely Enterococcus casseliflavus (Table 1).
None of the samples tested positive for viral nucleic acid or Clostridium difficile.
 DISCUSSION
DISCUSSION
This study has several limitations, such as the sample size, duration and number of participating nursing homes. However, the authors believe that this study gives an indication of the possible role that assistive technology and interactive therapeutic robots have in the transmission of microorganisms and that further research in this field is required to increase patient safety in nursing homes.
PARO robots are often used by several residents and shared between different nursing home sections. It is difficult to clean the artificial fur; it can only be removed and washed by the distributor. However, based on ATP monitoring and aerobic count (Fig. 3, a and b) it seems that bacteria do not long remain viable on the PARO. Nevertheless, it seems also that the PARO robot may be a beneficial environment for Staphylococcus aureus (Fig. 3, c). Further studies are needed to confirm this.
The finding that biological contamination in nursing home two was higher overall than in nursing home one, may be due to different managerial structures of the cleaning services. Cleaning in nursing home one is done by municipal employees only working in this particular nursing home, whereas cleaning personnel in nursing home two is done by employees working in different municipal institutions.
The presence of coliform bacteria (Table 1) on the fur of the PARO robot may be due to inadequate hand hygiene [9], and could indicate that the robot is contributing in the transfer of microorganisms between different patient zones.
The hygiene chairs showed a high level of bacterial contamination, even after standard cleaning. Interestingly, N1C1 which was not in use showed an increase in the aerobic count. This may indicate that the rough surface structure of the hygiene chairs may accumulate airborne bacteria. In general, this study has shown that current cleaning procedures for hygiene chairs are not adequate. One of the chairs in this study, N1C2, used by several residents, tested positive for cefotoxitin-resistant P. fulva and P. putida.
That influenza virus, norovirus and Clostridium difficile were not found may be due to unrelated factors. The national peak of influenza virus infections in Norway was in week 51 [10], three weeks before the first samples were taken. Furthermore, there were no ongoing infections in the nursing homes related to these agents and there have been only 46 clinical CDI cases in 2016 in the municipalities where the two nursing homes are located.
This study demonstrate the need for further research on the role of assistive technology and interactive therapeutic robots in pathogen distribution and the need for new cleaning procedures, a constant evaluation of infection control systems, as well as improved product design.
REFERENCES
1. Nakrem, S. and S. Jóhannes, Velferdsteknologi i praksis : perspektiver på teknologi i kommunal helse- og omsorgstjeneste. Velferdsteknologi i praksis. 2017, Oslo: Cappelen Damm akademisk.
2. Moyle, W., et al., Use of a Robotic Seal as a Therapeutic Tool to Improve Dementia Symptoms: A Cluster-Randomized Controlled Trial. J Am Med Dir Assoc, 2017.
3. Elstrøm, P., Smittevern i helseinstitusjoner. 2002, Oslo: Gyldendal akademisk.
4. Wertheim, H., et al., Improved detection of methicillin-resistant Staphylococcus aureus using phenyl mannitol broth containing aztreonam and ceftizoxime. J Clin Microbiol, 2001. 39(7): p. 2660-2.
5. Tunsjo, H.S., et al., A rapid, high-throughput screening method for carriage of methicillin-resistant Staphylococcus aureus. APMIS, 2013. 121(9): p. 865-70.
6. George, W.L., et al., Selective and differential medium for isolation of Clostridium difficile. J Clin Microbiol, 1979. 9(2): p. 214-9.
7. Mutters, R., et al., Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction.
J Hosp Infect, 2009. 71(1): p. 43-8.
8. Tunsjo, H.S., et al., Comparison of nasopharyngeal aspirate with flocked swab for PCR-detection of respiratory viruses in children. APMIS, 2015. 123(6): p. 473-7.
9. Messina, G., et al., Environmental contaminants in hospital settings and progress in disinfecting techniques. Biomed Res Int, 2013. 2013: p. 429780.
10. Nasjonalt folkehelseinstitutt Divisjon for, s., Influensasesongen i Norge 2016/2017 : årsrapport. Årsrapport, 2017.

